Chapter: 11th Physics : UNIT 8 : Heat and Thermodynamics
Zeroth Law of Thermodynamics
ZEROTH
LAW OF THERMODYNAMICS
The
zeroth law of thermodynamics states that if two systems, A and B, are in thermal
equilibrium with a third system, C,
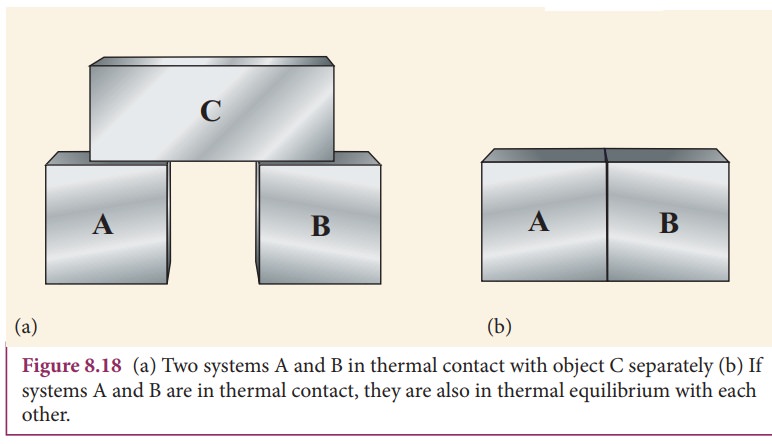
Consider three systems A, B and C which are initially at
different temperatures. Assume that A and B are not in thermal contact with
each other as shown in Figure 8.18 (a) but each of them is in thermal contact with
a third system C. After a lapse of time, system A will be in thermal
equilibrium with C and B also will be in thermal equilibrium with C. In this
condition, if the systems A and B are kept in thermal contact as shown in the
Figure 8.18 (b), there is no flow of heat between the systems A and B. It
implies that the system A and B are also in thermal equilibrium with each
other. Once the three systems are at thermal equilibrium, there will be no heat
flow between them as they are at the same temperature. This can be
mathematically expressed as
If TA = TC and TB = TC, it implies that TA = TB, where TA, TB and TC are the temperatures of the systems A, B, and C respectively.
Temperature is the property which determines whether the system is in thermal
equilibrium with other systems or not. Zeroth law enables us to determine the
temperature. For example, when a thermometer is kept in contact with a human
body, it reaches thermal equilibrium with the body. At this condition, the
temperature of the thermometer will be same as the human body. This principle
is used in finding the body temperature.
Related Topics