Thermodynamics - Cyclic processes and PV diagram for a cyclic process | 11th Physics : UNIT 8 : Heat and Thermodynamics
Chapter: 11th Physics : UNIT 8 : Heat and Thermodynamics
Cyclic processes and PV diagram for a cyclic process
Cyclic processes
This
is a thermodynamic process in which the thermodynamic system returns to its
initial state after undergoing a series of changes. Since the system comes back
to the initial state, the change in the internal energy is zero. In cyclic
process, heat can flow in to system and heat flow out of the system. From the
first law of thermodynamics, the net heat transferred to the system is equal to
work done by the gas.
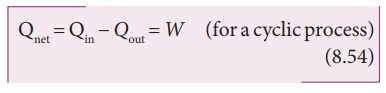
PV diagram for a cyclic process
In
the PV diagram the cyclic process is represented by a closed curve.
Let
the gas undergo a cyclic process in which it returns to the initial stage after
an expansion and compression as shown in Figure 8.39
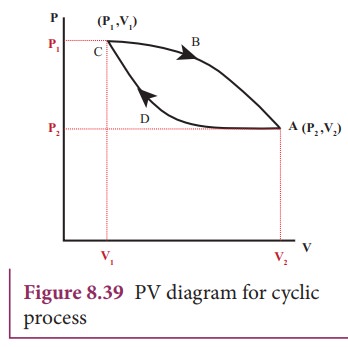
Let
W1 be the work done by the gas during expansion from volume V1
to volume V2. It is equal to area under the graph CBA as shown in
Figure 8.40 (a) .
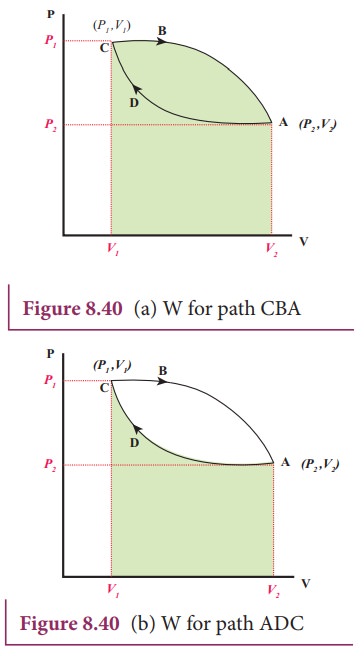
Let
W2 be the work done on the gas during compression from volume V2
to volume V1. It is equal to the area under the graph ADC as shown
in Figure 8.40 (b)
![]()
![]() The total work done in this cyclic process = W1 -
W2 = Green shaded area inside the loop, as shown in Figure 8.41.
The total work done in this cyclic process = W1 -
W2 = Green shaded area inside the loop, as shown in Figure 8.41.
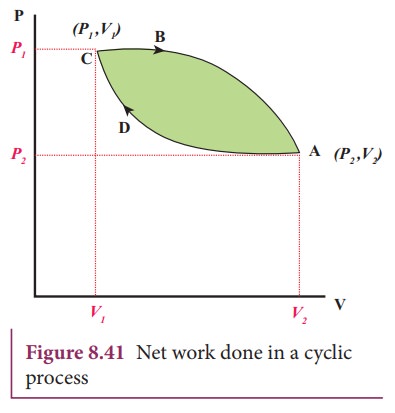
Thus
the net work done during the cyclic process shown above is not zero. In general
the net work done can be positive or negative. If the net work done is
positive, then work done by the system is greater than the work done on the
system. If the net work done is negative then the work done by the system is
less than the work done on the system.
EXAMPLE 8.22
The
PV diagrams for a thermodynamical system is given in the figure below.
Calculate the total work done in each of the cyclic processes shown.
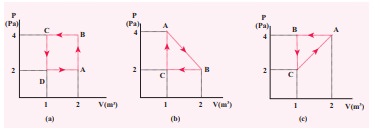
Solution
In
the case (a) the closed curve is anticlockwise. So the net work done is
negative, implying that the work done on the system is greater than the work
done by the system. The area under the curve BC will give work done on the gas
(isobaric compression) and area under the curve DA (work done by the system)
will give the total work done by the system.
Area
under the curve BC = Area of rectangle BC12 = 1 ├Ś 4= ŌłÆ 4J
Area
under the curve DA = 1 ├Ś 2= + 2J
Net
work done in cyclic process = ŌłÆ4 + 2= ŌłÆ2 J
In
the case (b) the closed curve is clockwise. So the net work done is positive,
implying that the work done on the system is less than the work done by the
system. Area under the curve BC will give work done on the gas (isobaric
compression) and area under the curve AB will give the total work done by the
system.
Area
under the curve AB = rectangle area+ triangle area = (1├Ś2) + 1/2 ├Ś
1├Ś2 = +3J
Area
under the curve BC = rectangle area = ![]()
![]() 1 ├Ś 2 = ŌłÆ 2J
1 ├Ś 2 = ŌłÆ 2J
Network
done in the cyclic process = 1 J, which is positive.
In
the case (c) the closed curve is anticlockwise. So the net work done is
negative, implying that the work done on the system is greater than work done
by the system. The area under the curve AB will give the work done on the gas
(isobaric compression) and area under the curve CA (work done by the system)
will give the total work done by the system.
The
area under the curve AB =Rectangle of area = 4 ├Ś 1 = - 4J
The
area under the curve CA = Rectangle area + triangle area = (1├Ś2) + 1/2 ├Ś 1├Ś2 =
+3J The total work in the cyclic
process = -1 J. It is negative
Related Topics