Chapter: 11th Physics : UNIT 8 : Heat and Thermodynamics
First law of thermodynamics
First law of thermodynamics
The first law of thermodynamics is a statement of the law of conservation of energy. In Newtonian mechanics conservation of energy involves kinetic and potential energies of bulk objects. But the first law of thermodynamics includes heat also. This law states that ŌĆśChange in internal energy (╬öU) of the system is equal to heat supplied to the system (Q) minus the work done by the system (W) on the surroundingsŌĆÖ. Mathematically it on the surroundingsŌĆÖ. Mathematically it is written as

The internal energy of a thermodynamic system can be changed either by heating or by work as shown below.
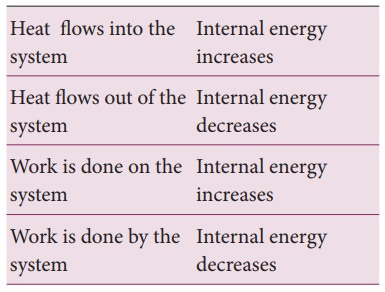
Based on the above table the sign convention is introduced to use first law of thermodynamics appropriately. It is shown in the following table and the Figure 8.20.
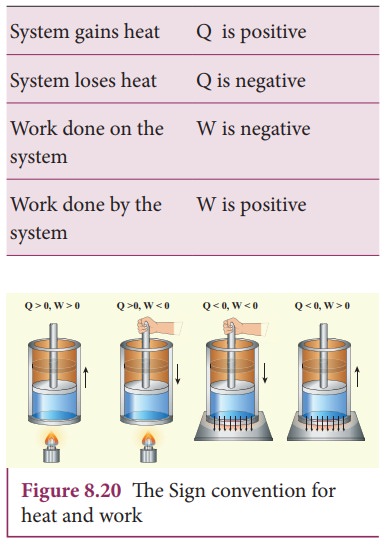
Even though we often explain first law of thermodynamics using gases, this law is universal and applies to liquids and solids also.
EXAMPLE 8.12
A person does 30 kJ work on 2 kg of water by stirring using a paddle wheel. While stirring, around 5 kcal of heat is released from water through its container to the surface and surroundings by thermal conduction and radiation. What is the change in internal energy of the system?
Solution
Work done on the system (by the person while stirring), W = -30 kJ = -30,000J
Heat flowing out of the system,
Q = -5 kcal = 5 ├Ś 4184 J =-20920 J
Using First law of thermodynamics
ŌłåU = Q-W
ŌłåU = -20,920 J-(-30,000) J
ŌłåU = -20,920 J+30,000 J = 9080 J
Here, the heat lost is less than the work done on the system, so the change in internal energy is positive.
EXAMPLE 8.13
Jogging every day is good for health. Assume that when you jog a work of 500 kJ is done and 230 kJ of heat is given off. What is the change in internal energy of your body?
Solution

Work done by the system (body),
W = +500 kJ
Heat released from the system (body),
Q = ŌĆō230 kJ
The change in internal energy of a body
= ╬öU= ŌĆō 230 kJ ŌĆō 500 kJ = ŌĆō 730 kJ
Related Topics