Chapter: 11th Physics : UNIT 8 : Heat and Thermodynamics
Reversible process - Thermodynamics
Reversible
process
A
thermodynamic process can be considered reversible only if it possible to
retrace the path in the opposite direction in such a way that the system and
surroundings pass through the same states as in the initial,direct process.
Example:
A quasi–static isothermal expansion of gas, slow compression and expansion of a
spring. Conditions for reversible process:
1.
The process should proceed at an extremely slow rate.
2.
The system should remain in mechanical, thermal and chemical equilibrium state
at all the times with the surroundings, during the process.
3.
No dissipative forces such as friction, viscosity, electrical resistance should
be present.
Irreversible process:
All
natural processes are irreversible. Irreversible process cannot be plotted in a
PV diagram, because these processes cannot have unique values of pressure,
temperature at every stage of the process.
The
first law of thermodynamics is the statement about conservation of energy in a
thermodynamic process. For example, if a hotter object is placed on a colder
object, heat flows from hotter to colder object. Why does heat not flow from
the colder object to hotter object? Even if energy flows from colder object to
hotter object, the first law of thermodynamics is not violated. For example, if
5 J of heat flows form hotter to colder or from colder to hotter objects the
total internal energy of this combined system remains the same. But 5 J of heat
never flows from the colder object to hotter object. In nature all such process
occur only in one direction but not in the reverse direction, even if the
energy is conserved in both the processes. Thus the first law of thermodynamics
has no explanation for this irreversibility. When the scientists of the
eighteenth century tried to explain this irreversibility, they discovered a new
law of nature. This is called the second law of thermodynamics. According to
second law of thermodynamics
![]()
![]() “Heat always flows from hotter object to colder object
spontaneously”. This is known as the Clausius form of second law of
thermodynamics.
“Heat always flows from hotter object to colder object
spontaneously”. This is known as the Clausius form of second law of
thermodynamics.
EXAMPLE 8.23
Give
some examples of irreversible processes.
All
naturally occuring processes are irreversible. Here we give some interesting
examples.
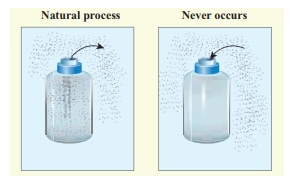
(a)
When we open a gas bottle, the gas molecules slowly spread into the entire
room. These gas molecules can never get back in to the bottle.
(b) Suppose one drop of an
ink is dropped
in water, the ink
droplet slowly spreads in the water. It is impossible to get the ink droplet
back.
(c) When an object falls
from some
height, as soon as it
hits the earth it comes to rest. All the kinetic energy of the object is
converted to kinetic energy of molecules of the earth surface, molecules of the
object and small amount goes as sound energy. The spreaded kinetic energy to
the molecules never collected back and object never goes up by itself.
Note
that according to first law of thermodynamics all the above processes are
possible in both directions. But second law of thermodynamics forbids The
processes to occur in the reverse direction. The second law of thermodynamics
is one of the very important laws of nature. It controls the way the natural
processes occur.
Related Topics