Chapter: 11th Physics : UNIT 8 : Heat and Thermodynamics
Solved Example Problems for Calorimetry
EXAMPLE 8.7
If 5 L of water at 50┬░C is mixed with 4L of water at 30┬░C, what will be the final temperature of water? Take the specific heat capacity of water as 4184 J kg-1K-1.
Solution
We can use the equation
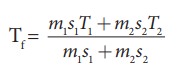
m1 = 5L = 5kg and m2= 4L = 4kg, s1 = s2 and T1=50┬░C =323K and T2 = 30┬░C=303 K.
So
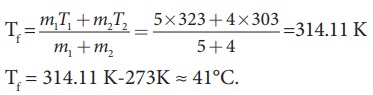
Tf = 314.11 K-273K Ōēł 41┬░C.
Suppose if we mix equal amount of water (m1 = m2) with 50┬░C and 30┬░C, then the final temperature is average of two temperatures.
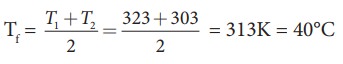
Suppose if both the water are at 30┬░C then the final temperature will also 30┬░C. It implies that they are at equilibrium and no heat exchange takes place between each other.
Related Topics