Thermodynamics - Refrigerator | 11th Physics : UNIT 8 : Heat and Thermodynamics
Chapter: 11th Physics : UNIT 8 : Heat and Thermodynamics
Refrigerator
REFRIGERATOR
A
refrigerator is a Carnot’s engine working in the reverse order. It is shown in
the figure 8.49.
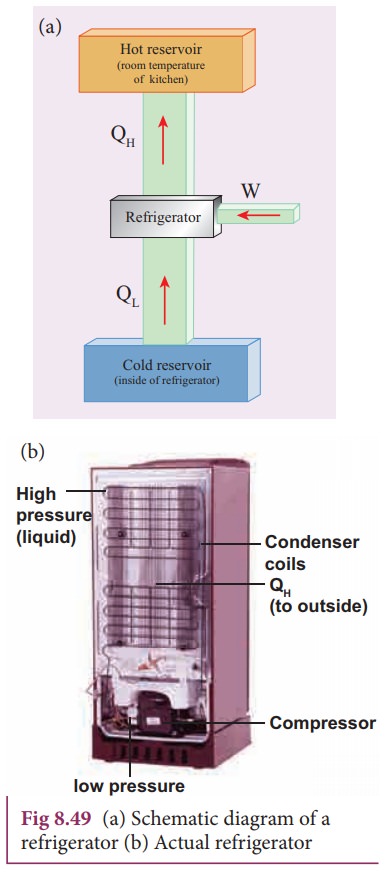
Working Principle:
The working substance (gas) absorbs a quantity of heat QL from the cold body (sink)
at a lower temperature TL.
A certain amount of work W is done on the working substance by the compressor
and a quantity of heat QH is rejected to the hot body (source) ie,
the atmosphere at TH. When you stand beneath of refrigerator, you
can feel warmth air.
From
the first law of thermodynamics , we have

As
a result the cold reservoir (refrigerator) further cools down and the
surroundings (kitchen or atmosphere) gets hotter.
Coefficient of performance (COP) (β):
COP
is a measure of the efficiency of a refrigerator. It is defined as the ratio of
heat extracted from the cold body (sink) to the external work done by the
compressor W.
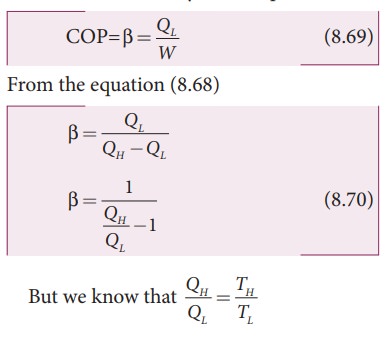
Substituting
this equation into equation (8.70) we get
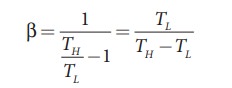
Inferences:
1.
The greater the COP, the better is the condition of the refrigerator. A typical
refrigerator has COP around 5 to 6.
2.
Lesser the difference in the temperatures of the cooling chamber and the
atmosphere, higher is the COP of a refrigerator.
3.
In the refrigerator the heat is taken from cold object to hot object by doing
external work. Without external work heat cannot flow from cold object to hot
object. It is not a violation of second law of thermodynamics, because the heat
is ejected to surrounding air and total entropy of (refrigerator + surrounding)
is always increased.
EXAMPLE 8.27
A
refrigerator has COP of 3. How much work must be supplied to the refrigerator
in order to remove 200 J of heat from its interion?
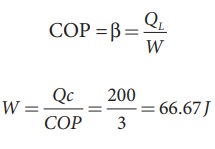
Related Topics