Chapter: 11th Physics : UNIT 8 : Heat and Thermodynamics
Multiple Choice Questions - Physics: Heat and Thermodynamics
Heat and Thermodynamics (Physics)
Multiple choice questions:
1. In hot summer after a bath, the bodyŌĆÖs
a) internal energy decreases
b) internal energy increases
c) heat decreases
d) no change in internal energy and heat
Answer : a) internal energy decreases
Solution : Temperature decreases, So internal energy decreases.
2. The graph between volume and temperature in CharlesŌĆÖ law is
a) an ellipse
b) a circle
c) a straight line
d) a parabola
Answer : c) a straight line
Solution: P - constant, VŌłØT
3. When a cycle tyre suddenly bursts, the air inside the tyre expands. This process is
a) isothermal
b) adiabatic
c) isobaric
d) isochoric
Answer : b) adiabatic
4. An ideal gas passes from one equilibrium state (P1, V1, T1, N) to another equilibrium state (2P1, 3V1, T2, N). Then
a) T1=T2
b) T1=T2/6
c) T1=6T2
d) T1=3T2
Answer: b) T1 = T2 / 6
Solution:
T1 = T2 / 6
2P1 3V1 = NKT2
6P1V1 = NKT2
P1V1 = NKT2 / 6
NKT1 = NK T2 / 6
P1V1 = NKT1
NKT1 = NK T2 / 6
T1 = T2 / 6
5. When a uniform rod is heated, which of the following quantity of the rod will increase
a) mass
b) weight
c) center of mass
d) moment of inertia
Answer : d) moment of inertia
Solution:
I = mr2
Due to thermal expansion, if T increases I also increases.
6. When food is cooked in a vessel by keeping the lid closed, after some time the steam pushes the lid outward. By considering the steam as a thermodynamic system, then in the cooking process
a) Q > 0, W > 0,
b) Q < 0, W > 0,
c) Q > 0, W < 0,
d) Q < 0, W < 0,
Answer: a) Q>0,W>0,
Solution:
Heat is given to the system
System gains heat ŌåÆ Q > 0
Lid Pushes upwards ŌåÆ Work done
by the system ŌåÆ W > 0
7. When you exercise in the morning, by considering your body as thermodynamic system, which of the following is true?
a) U > 0, W > 0,
b) U < 0, W > 0,
c) U < 0, W < 0,
d) U = 0, W > 0,
Answer : b) ΔU < 0, W > 0,
Solution:
Workdone by the system, W > 0
Heat flows outward ŌåÆ ╬öU Ōåō
8. A hot cup of coffee is kept on the table. After some time it attains a thermal equilibrium with the surroundings. By considering the air molecules in the room as a thermodynamic system, which of the following is true
a) U > 0, Q = 0
b) U > 0, W < 0
c) U > 0, Q > 0
d) U = 0, Q > 0
Answer : c) ΔU > 0, Q
> 0
9. An ideal gas is taken from (Pi,Vi) to (Pf,Vf) in three different ways. Identify the process in which the work done on the gas the most.
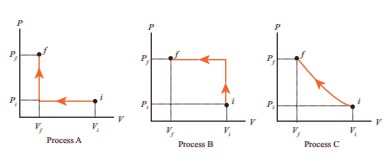
a) Process A
b)Process B
c) Process C
d) Equal work is done in Process A,B &C
Answer : b) Process B
10. The V-T diagram of an ideal gas which goes through a reversible cycle AŌåÆBŌåÆCŌåÆD is shown below. (Processes DŌåÆA and BŌåÆC are adiabatic)
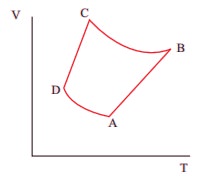
The corresponding PV diagram for the process is (all figures are schematic)
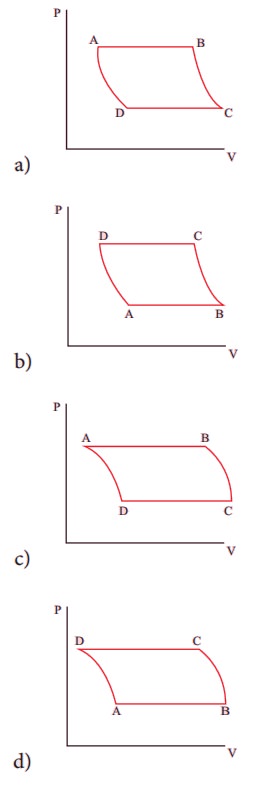
Ans: b
11. A distant star emits radiation with maximum intensity at 350 nm. The temperature of the star is
a) 8280 K
b) 5000K
c) 7260 K
d) 9044 K
Answer: a) 8280 K
Solution:
╬╗m = b / T
ŌćÆ T = b / ╬╗m
= 2.898├Ś10-3 / 350├Ś10-9
╬╗m = 8280 K
12. Identify the state variables given here?
a) Q, T, W
b) P, T, U
c) Q, W
d) P, T, Q
Answer : b) P, T, U
13. In an isochoric process, we have
a) W = 0
b) Q = 0
c) ŌłåU = 0
d) ŌłåT = 0
Answer: a) W = 0
14. The efficiency of a heat engine working between the freezing point and boiling point of water is
a) 6.25%
b) 20%
c) 26.8%
d) 12.5%
Answer: c) 26.8%
Solution:
Tn = 100┬░C
Tn = 373
TL = 0┬░C
TL = 273 K
[ TH ŌłÆ TL ] / TH = n
= ( [373 ŌĆō 273] / 373 ) ├Ś 100
= ( 100 / 373 ) ├Ś 100
n = 26.8%,
15. An ideal refrigerator has a freezer at temperature ŌłÆ12┬░C. The coefficient of performance of the engine is 5. The temperature of the air (to which the heat ejected) is
(a) 50┬░C
(b) 45.2┬░C
(c) 40.2┬░C
(d)37.5┬░C
Answer : c) 40.2┬░C
Solution:
TL = ŌłÆ12┬░C + 278 K
TL = 261 K,
B=5,
TH = ?
╬▓ = TL / [TH
ŌĆō TL ]
ŌćÆ TH = [ TL
/ ╬▓ ] + TL
= [261/5] + 261
= 525 + 261
= 313.2K
TH = 313.2 K ŌłÆ
273┬░C
TH = 40.2┬░C
Answers:
1) a 2) c 3) b 4) b
5) d 6) a 7) b 8) c
9) b 10) b 11) a 12) b
13) a 14) b 15) c
Related Topics