Chapter: 11th Physics : UNIT 8 : Heat and Thermodynamics
Heat capacity and specific heat capacity - Thermal Properties of Matter
Heat
capacity and specific heat capacity
Take
equal amount of water and oil at temperature 27┬░C and heat both of them till
they reach the temperature 50┬░C. Note down the time taken by the water and oil
to reach the temperature 50┬░C. Obviously these times are not same. We can see
that water takes more time to reach 50┬░C than oil. It implies that water
requires more heat energy to raise its temperature than oil. Now take twice the
amount of water at 27┬░C and heat it up to 50┬░C , note the time taken for this
rise in temperature. The time taken by the water is now twice compared to the
previous case.
We
can define ŌĆśheat capacityŌĆÖ as the amount of heat energy required to raise the
temperature of the given body from T to T + ŌłåT .
Heat
capacity S = ŌłåQ / ŌłåT
Specific heat capacity of a
substance is defined as the amount of heat energy required to raise the
temperature of 1kg of a substance by 1 Kelvin or 1┬░C
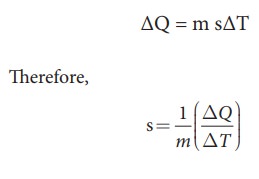
Where
s is known as specific heat capacity
of a substance and its value depends only on the nature of the substance not
amount of substance
ΔQ
= Amount of heat energy
ΔT
= Change in temperature
m
= Mass of the substance
The
SI unit for specific heat capacity is J
kg-1K-1.
Heat capacity and specific heat capacity
are always positive quantities.
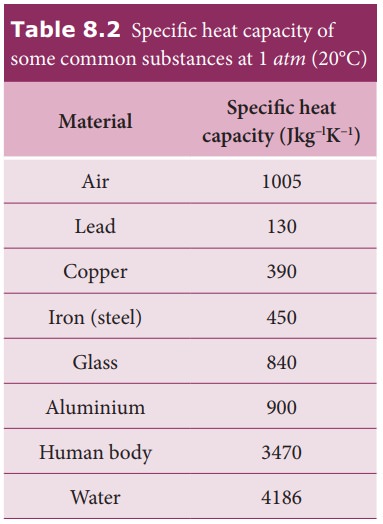
From the table it is clear that water has the highest value of specific heat capacity. For this reason it is used as a coolant in power stations and reactors.
When
we study properties of gases, it is more practical to use molar specific heat
capacity. Molar specific heat capacity is defined as heat energy required to
increase the temperature of one mole of substance by 1K or 1┬░C. It can be
written as follows
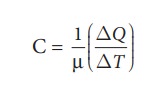
Here
C is known as molar specific heat capacity of a substance and ╬╝ is number
of moles in the substance.
The
SI unit for molar specific heat capacity is J
mol-1K-1 .
It is also a positive quantity.
Related Topics