Thermodynamics - Adiabatic process | 11th Physics : UNIT 8 : Heat and Thermodynamics
Chapter: 11th Physics : UNIT 8 : Heat and Thermodynamics
Adiabatic process
Adiabatic
process
This
is a process in which no heat flows into or out of the system (Q=0). But the
gas can expand by spending its internal energy or gas can be compressed through
some external work. So the pressure, volume and temperature of the system may
change in an adiabatic process.
For
an adiabatic process, the first law becomes ŌłåU = W.
This
implies that the work is done by the gas at the expense of internal energy or
work is done on the system which increases its internal energy.
The
adiabatic process can be achieved by the following methods
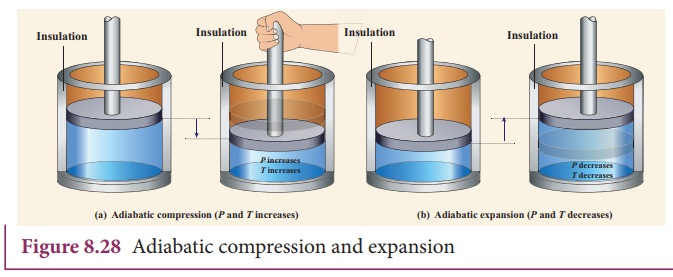
(i)
Thermally insulating the system from surroundings so that no heat flows into or
out of the system; for example, when thermally insulated cylinder of gas is
compressed (adiabatic compression) or expanded (adiabatic expansion) as shown
in the Figure 8.28
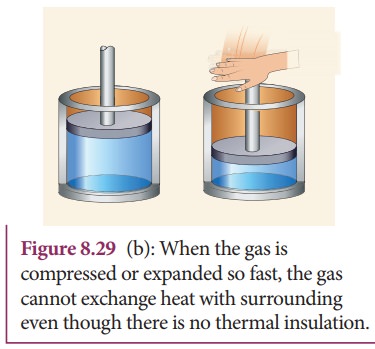
(ii)
If the process occurs so quickly that there is no time to exchange heat with
surroundings even though there is no thermal insulation. A few examples are
shown in Figure 8.29.
![]()
![]()
Examples:


The
equation of state for an adiabatic process is given by
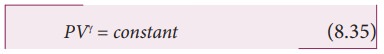
Here
╬│ is called adiabatic exponent (╬│ = Cp/Cv) which depends on the
nature of the gas.
The
equation (8.35) implies that if the gas goes from an equilibrium state (Pi,Vi) to another
equilibrium state (PfŌĆē,Vf)
adiabatically then it satisfies the relation

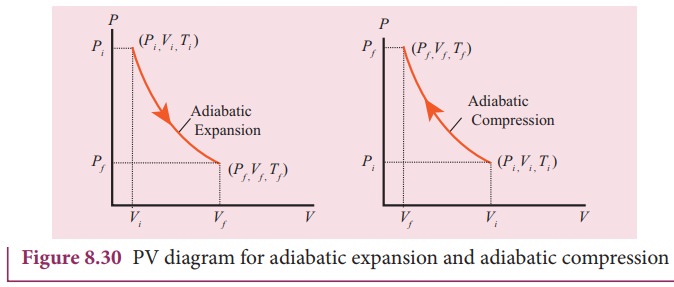
The
PV diagram of an adiabatic expansion and adiabatic compression process are
shown in Figure 8.30. The PV diagram for an adiabatic process is also called adiabat Note that the PV diagram for
isothermal (Figure 8.25) and adiabatic (Figure 8.30) processes look similar.
But actually the adiabatic curve is steeper than isothermal curve.
![]()
![]() We can also rewrite the equation (8.35) in terms of T and V.
From ideal gas equation, the pressure P
= ╬╝RT/V. Substituting this equation in the equation (8.35), we have
We can also rewrite the equation (8.35) in terms of T and V.
From ideal gas equation, the pressure P
= ╬╝RT/V. Substituting this equation in the equation (8.35), we have
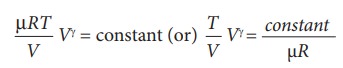
Note
here that is another constant. So it can be written as

The
equation (8.37) implies that if the gas goes from an initial equilibrium state
(Ti,ŌĆŖVi) to final equilibrium state (Tf,ŌĆŖVf) adiabatically then it satisfies
the relation
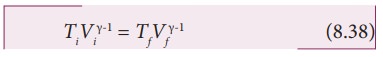
The
equation of state for adiabatic process can also be written in terms of T and P
as

(The
proof of equation (8.39) left as an exercise).
EXAMPLE 8.18

We
often have the experience of pumping air into bicycle tyre using hand pump.
Consider the air inside the pump as a thermodynamic system having volume V at
atmospheric pressure and room temperature, 27┬░C. Assume that the nozzle of the
tyre is blocked and you push the pump to a volume 1/4 of V. Calculate the final
temperature of air in the pump? (For air , since the nozzle is blocked air will
not flow into tyre and it can be treated as an adiabatic compression).
Solution
Here,
the process is adiabatic compression. The volume is given and temperature is to
be found. we can use the equation (8.38 )
TiVi╬│-1 = TfVf╬│-1.
Ti = 300 K (273+27┬░C = 300 K)
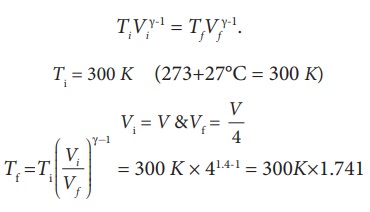
T2
Ōēł 522 K or 2490C
This temperature is higher than the boiling point of water. So it is very dangerous to touch the nozzle of blocked pump when you pump air.
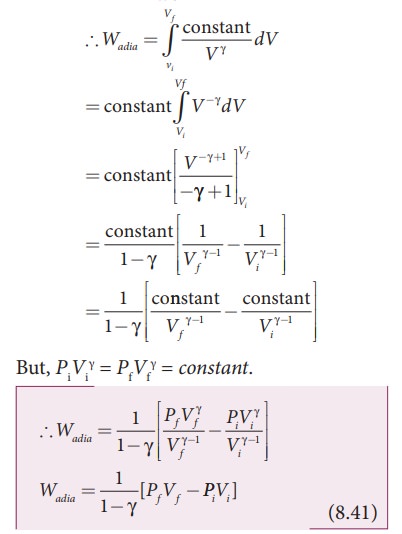
Work done in an adiabatic process:
Consider
╬╝ moles of an ideal gas enclosed in a cylinder having perfectly non conducting
walls and base. A frictionless and insulating piston of cross sectional area A
is fitted in the cylinder as shown in Figure 8.31.
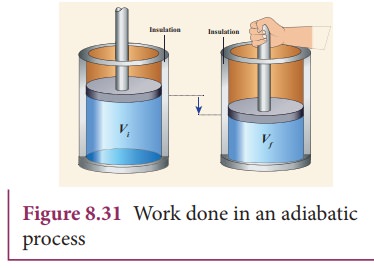
Let
W be the work done when the system goes from the initial state (Pi,Vi,Ti)
to the final state (Pf,Vf,Tf) adiabatically.

By
assuming that the adiabatic process occurs quasi-statically, at every stage the
ideal gas law is valid. Under this condition, the adiabatic equation of state
is PV╬│=constant (or)
P
= constant/V╬│ can be
substituted in the equation (8.40), we get
From
ideal gas law,
PfVf = ╬╝RTf and
PiVi = ╬╝RTi
Substituting
in equation (8.41), we get

In
adiabatic expansion, work is done by the gas. i.e., Wadia is positive. As Ti>Tf
, the gas cools during adiabatic expansion.
In
adiabatic compression, work is done on the
gas. i.e., Wadia is
negative. As Ti<Tf, the temperature of the
gas increases during adiabatic compression.
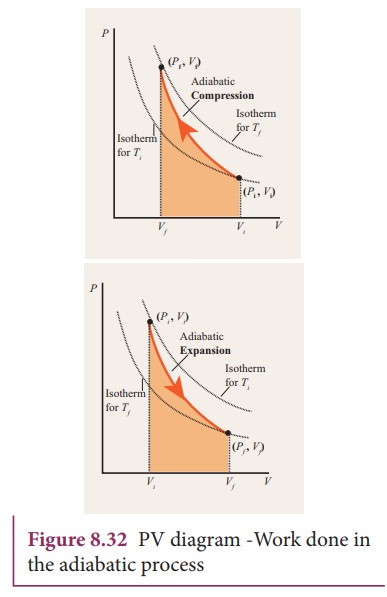
To
differentiate between isothermal and adiabatic curves in (Figure 8.32) the
adiabatic curve is drawn along with isothermal curve for Tf and Ti.
Note that adiabatic curve is steeper than isothermal curve. This is because ╬│
> 1 always.
Related Topics