Chapter: 11th Physics : UNIT 8 : Heat and Thermodynamics
Thermodynamics
THERMODYNAMICS:
Introduction
In
the previous sections we have studied about the heat, temperature and thermal
properties of matter. Thermodynamics is a
branch of physics which describes the laws governing the process of conversion
of work into heat and conversion of heat into work. The laws of thermodynamics
are formulated over three centuries of experimental works of Boyle, Charles,
Bernoulli, Joule, Clausius, Kelvin, Carnot and Helmholtz. In our day to day
life, the functioning of everything around us and even our body is governed by
the laws of thermodynamics. Therefore thermodynamics is one of the essential
branches of physics.
Thermodynamic system:
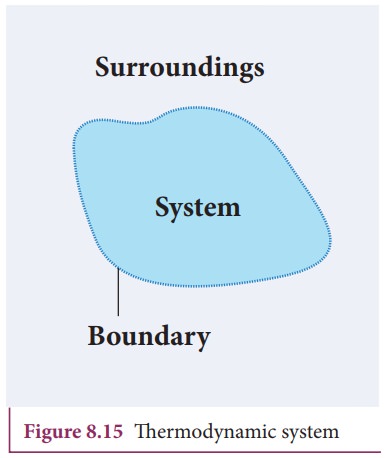
A
thermodynamic system is a finite part of the universe. It is a collection of
large number of particles (atoms and molecules) specified by certain parameters
called pressure (P), Volume (V) and Temperature (T). The remaining part of the
universe is called surrounding. Both are separated by a boundary. It is shown
in Figure 8.15
Examples:
![]()
![]() A thermodynamic system can be liquid, solid, gas and
radiation.
A thermodynamic system can be liquid, solid, gas and
radiation.
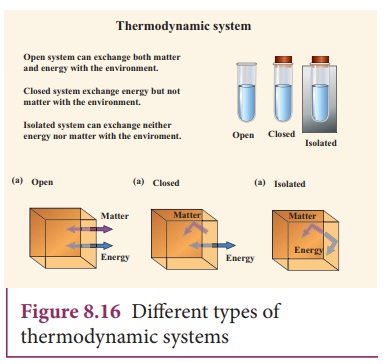
We
can classify thermodynamics system into three types: It is given in Figure 8.16
Thermal equilibrium
When
a hot cup of coffee is kept in the room, heat flows from coffee to the
surrounding air. After some time the coffee reaches the same temperature as the
surrounding air and there will be no heat flow from coffee to air or air to
coffee. It implies that the coffee and surrounding air are in thermal
equilibrium with each other.
Two
systems are said to be in thermal equilibrium with each other if they are at
the same temperature, which will not change with time.
Mechanical equilibrium:
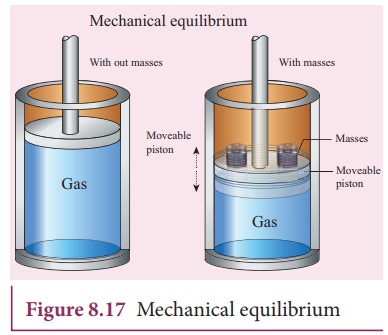
Consider
a gas container with piston as shown in Figure 8.17. When some mass is placed
on the piston, it will move downward due to downward gravitational force and
after certain humps and jumps the piston will come to rest at a new position.
When the downward gravitational force given by the piston is balanced by the
upward force exerted by the gas, the system is said to be in mechanical equilibrium.
A system is said to be in mechanical equilibrium if no unbalanced force acts on
the thermo dynamic system or on the surrounding by thermodynamic system.
Chemical equilibrium:
If
there is no net chemical reaction between two thermodynamic systems in contact
with each other then it is said to be in chemical equilibrium.
Thermodynamic equilibrium:
If
two systems are set to be in thermodynamic equilibrium, then the systems are at
thermal, mechanical and chemical equilibrium with each other. In a state of
thermodynamic equilibrium the macroscopic variables such as pressure, volume
and temperature will have fixed values and do not change with time.
Thermodynamic state variables
In
mechanics velocity, momentum and acceleration are used to explain the state of
any moving object (which you would have realized in Volume 1). In
thermodynamics, the state of a thermodynamic system is represented by a set of
variables called thermodynamic variables.
Examples:
Pressure, temperature, volume and internal energy etc.
The
values of these variables completely describe the equilibrium state of a
thermodynamic system. Heat and work are not state variables rather they are
process variables.
There
are two types of thermodynamic variables: Extensive and Intensive Extensive
variable depends on the size or mass of the system.
Example: Volume, total mass, entropy, internal energy, heat capacity etc.
Intensive
variables do not depend on the size or mass of the system.
Example: Temperature, pressure, specific heat capacity, density etc.
Equation of state:
The equation which connects the state variables in a specific manner is called equation of state. A thermodynamic equilibrium is completely specified by these state variables by the equation of state. If the system is not in thermodynamic equilibrium then these equations cannot specify the state of the system.
An ideal gas obeys the equation PV = NkT at thermodynamic
equilibrium. Since all four macroscopic variables (P,V,T and N) are connected
by this equation, we cannot change one variable alone. For example, if we push
the piston of a gas container, the volume of the gas will decrease but pressure
will increase or if heat is supplied to the gas, its temperature will increase,
pressure and volume of the gas may also increase.
There
is another example of equation of state called van der Waals equation. Real
gases obey this equation at thermodynamic equilibrium. The air molecules in the
room truly obey van der Waals equation of state. But at room temperature with
low density we can approximate it into an ideal gas.
Related Topics