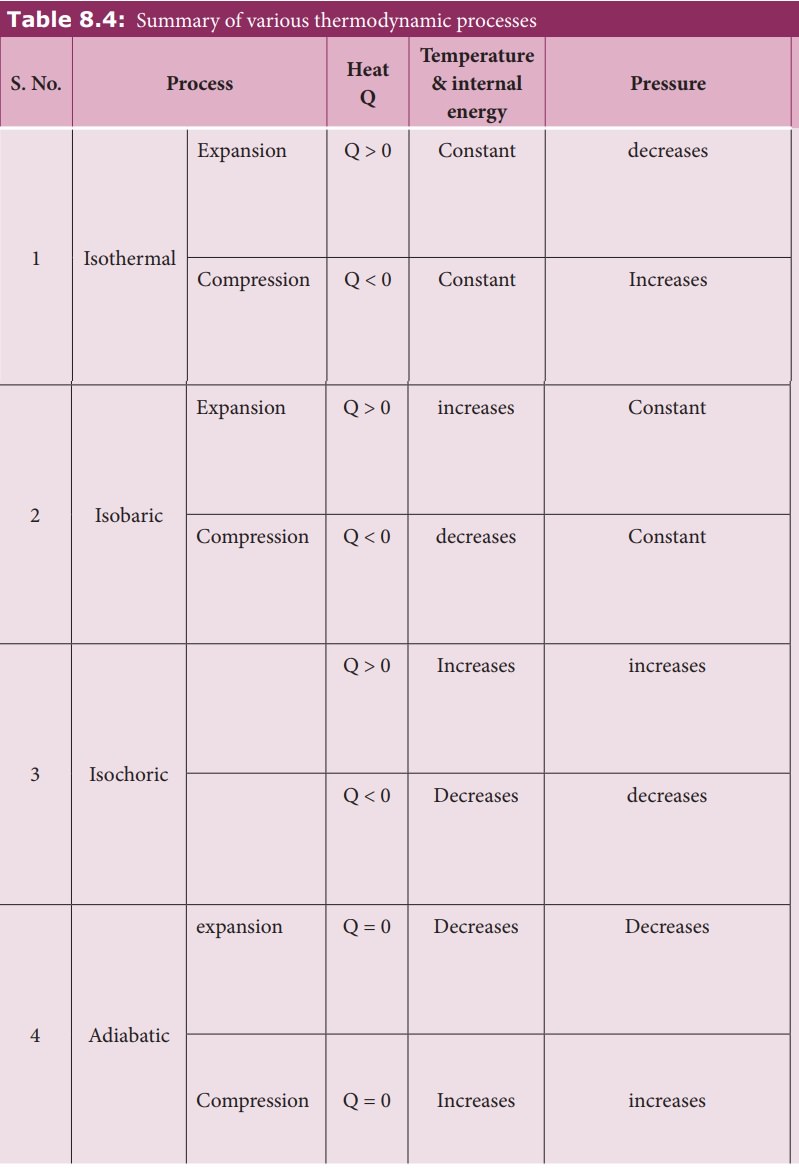
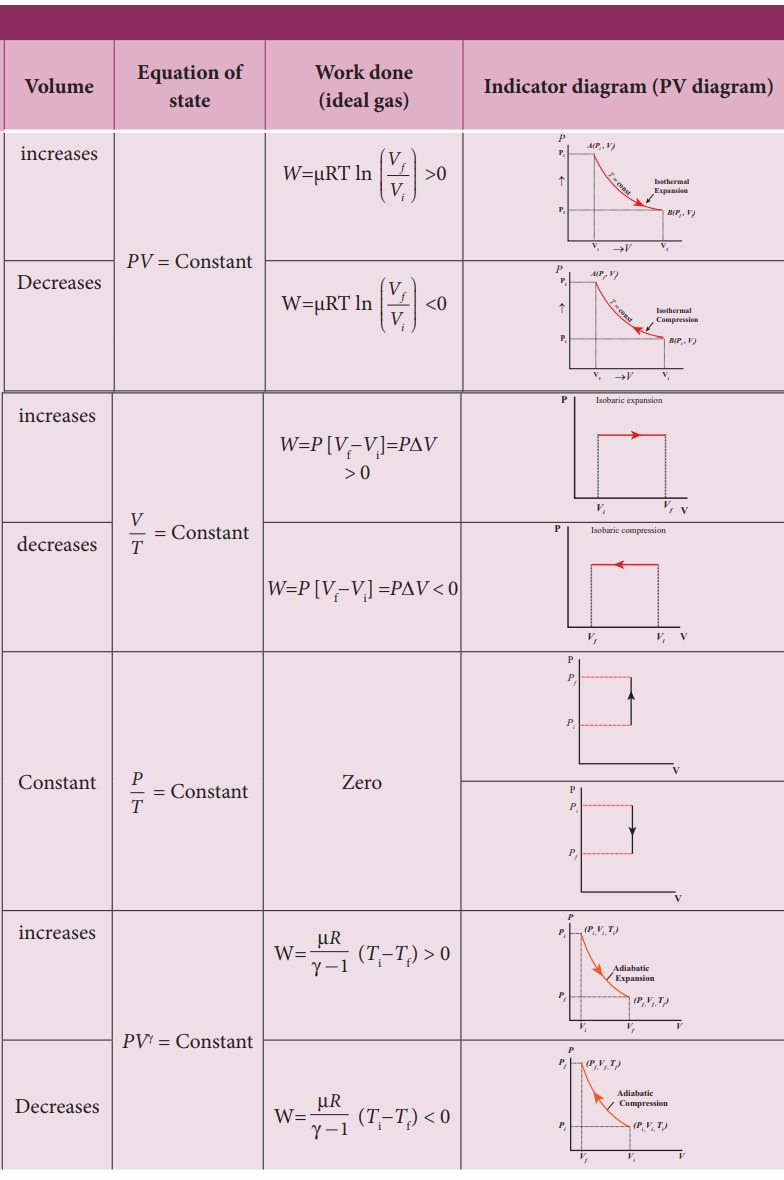
Thermodynamics - Isochoric process | 11th Physics : UNIT 8 : Heat and Thermodynamics
Chapter: 11th Physics : UNIT 8 : Heat and Thermodynamics
Isochoric process
Isochoric
process
This
is a thermodynamic process in which the volume of the system is kept constant.
But pressure, temperature and internal energy continue to be variables.
The
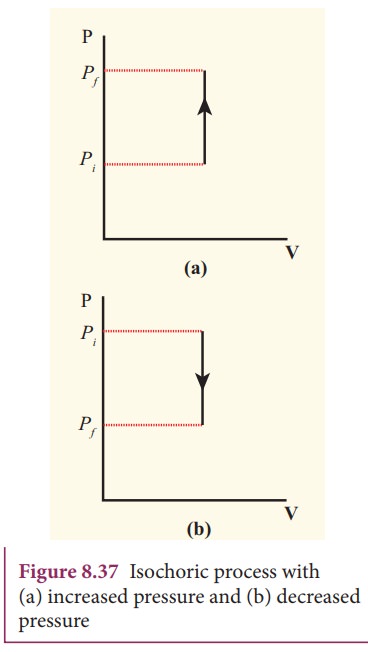
pressure - volume graph for an isochoric process is a vertical line parallel to
pressure axis as shown in Figure 8.37.
The
equation of state for an isochoric process is given by
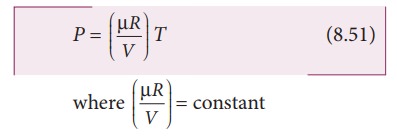
We
can infer that the pressure is directly proportional to temperature. This
implies that the P-T graph for an isochoric process is a straight line passing
through origin.
If
a gas goes from state (Pi,Ti) to (Pf,Tf)
at constant volume, then the system satisfies the following equation

For
an isochoric processes, ŌłåV=0 and W=0.
Then
the first law becomes

Implying
that the heat supplied is used to increase only the internal energy. As a
result the temperature increases and pressure also increases. This is shown in
Figure 8.38
Suppose
a system loses heat to the surroundings through conducting walls by keeping the
volume constant, then its internal energy decreases. As a result the
temperature decreases; the pressure also decreases.
Examples:
1.
When food is cooked by closing with a lid as shown in figure.

When
food is being cooked in this closed position, after a certain time you can
observe the lid is being pushed upwards by the water steam. This is because
when the lid is closed, the volume is kept constant. As the heat continuously
supplied, the pressure increases and water steam tries to push the lid upwards.
2.
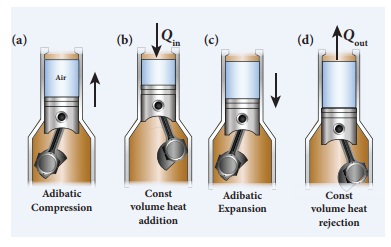
In automobiles the petrol engine undergoes four processes. First the piston is
adiabatically compressed to some volume as shown in the Figure (a). In the
second process (Figure (b)), the volume of the air-fuel mixture is kept
constant and heat is being added. As a result the temperature and pressure are
increased. This is an isochoric process. For a third stroke (Figure (c)) there
will be an adiabatic expansion, and fourth stroke again isochoric process by
keeping the piston immoveable (Figure (d)).
The
summary of various thermodynamic processes is give in the Table 8.4.
EXAMPLE 8.21
500
g of water is heated from 30┬░C to 60┬░C. Ignoring the slight expansion of water,
calculate the change in internal energy of the water? (specific heat of water
4184 J/kg.K)
Solution
When
the water is heated from 30┬░C to 60┬░C,there is only a slight change in its
volume. So we can treat this process as isochoric. In an isochoric process the
work done by the system is zero. The given heat supplied is used to increase
only the internal energy.
ŌłåU
= Q = msv ŌłåT
The
mass of water = 500 g =0.5 kg
The
change in temperature = 30K
The
heat Q = 0.5├Ś4184├Ś30 = 62.76 kJ
Related Topics