Chapter: 11th Physics : UNIT 8 : Heat and Thermodynamics
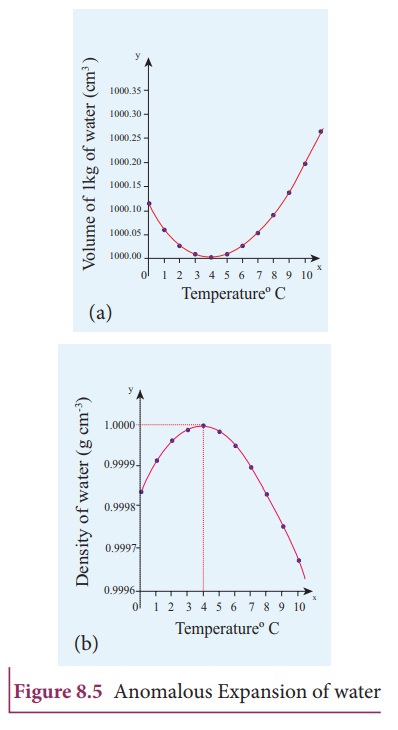
Anomalous expansion of water
Anomalous
expansion of water
Liquids
expand on heating and contract on cooling at moderate temperatures. But water
exhibits an anomalous behavior. It contracts on heating between 0ËšC and 4ËšC.
The volume of the given amount of water decreases as it is cooled from room
temperature, until it reach 4ËšC . Below 4ËšC the volume increases and so the
density decreases. This means that the water has a maximum density at 4ËšC .
This behavior of water is called anomalous expansion of water. It is shown in
the Figure 8.5.
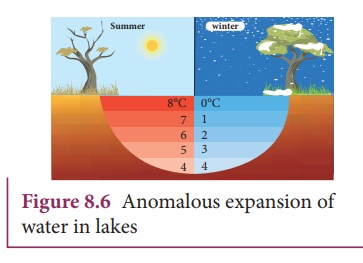
In cold countries during the winter season, the surface of the lakes will be at lower temperature than the bottom as shown in the Figure 8.6. Since the solid water (ice) has lower density than its liquid form, below 4°C, the frozen water will be on the top surface above the liquid water (ice floats).
This is due to the
anomalous expansion of water. As the water in lakes and ponds freeze only at
the top the species living in the lakes will be safe at the bottom.
Related Topics