Introduction - Electrostatics | 12th Physics : Electrostatics
Chapter: 12th Physics : Electrostatics
Electrostatics
ELECTROSTATICS
INTRODUCTION
Electromagnetism is one
of the most important branches of physics. The technological developments of
the modern 21st century are primarily due to our understanding of
electromagnetism. The forces we experience in everyday life are electromagnetic
in nature except gravity.
In standard XI, we
studied about the gravitational force, tension, friction, normal force etc.
Newton treated them to be independent of each other with each force being a
separate natural force. But what is the origin of all these forces? It is now
understood that except gravity, all forces which we experience in every day
life (tension in the string, normal force from the surface, friction etc.)
arise from electromagnetic forces within the atoms. Some examples are
(i) When an object is
pushed, the atoms in our hand interact with the atoms in the object and this
interaction is basically electromagnetic in nature.
(ii) When we stand on
Earth's surface, the gravitational force on us acts downwards and the normal
force acts upward to counter balance the gravitational force. What is the
origin of this normal force?
It arises due to the
electromagnetic interaction of atoms on the surface of the Earth with the atoms
present in the feet of the person. Though, we are attracted by the
gravitational force of the Earth, we stand on Earth only because of
electromagnetic force of atoms.
(iii) When an object is
moved on a surface, static friction resists the motion of the object. This
static friction arises due to electromagnetic interaction between the atoms
present in the object and atoms on the surface. Kinetic friction also has
similar origin.
From these examples,
it is clear that understanding
electromagnetism is very essential to understand the universe in a holistic manner.
The basic principles of electromagnetism are dealt in XII physics volume 1.
This unit deals with the behaviour and other related phenomena of charges at
rest. This branch of electricity which deals with stationary charges is
called Electrostatics.
1. Historical background of electric charges
Two millenniums ago,
Greeks noticed that amber (a solid, translucent material formed from the resin
of a fossilized tree) after rubbing with animal fur attracted small pieces of
leaves and dust. The amber possessing this property is said to be ‘charged’. It
was initially thought that amber has this special property. Later people found
that not only amber but even a glass rod rubbed with silk cloth, attracts
pieces of papers. So glass rod also becomes ‘charged’ when rubbed with a
suitable material.![]()
![]()
Consider a charged
rubber rod hanging from a thread as shown in Figure 1.1. Suppose another
charged rubber rod is brought near the first rubber rod; the rods repel each
other. Now if we bring a charged glass rod close to the charged rubber rod,
they attract each other. At the same time, if a charged glass rod is brought
near another charged glass rod, both the rods repel each other.
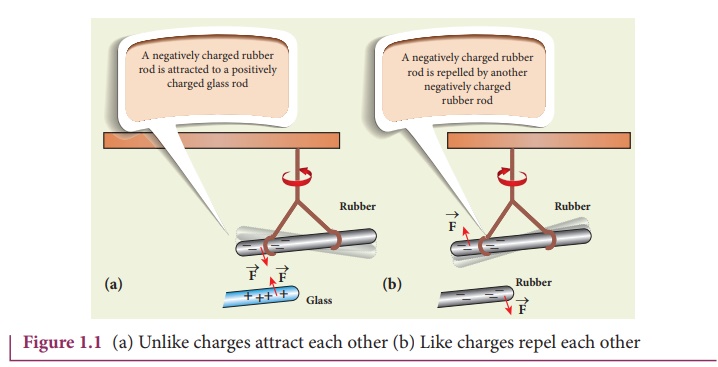
From these observations,
the following inferences are made
i.
The charging of rubber rod and that of glass rod are different
from one another.
ii.
The charged rubber rod repels another charged rubber rod, which
implies that ‘like charges repel each other’. We can also arrive at the same
inference by observing that a charged glass rod repels another charged glass
rod.
iii.
The charged amber rod attracts the charged glass rod, implying
that the charge in the glass rod is not the same kind of charge present
in the rubber.
Thus unlike charges attract each other. Therefore, two kinds of charges exist in the universe. In the 18th century, Benjamin Franklin called one type of charge as positive (+) and another type of charge as negative (-). Based on Franklin’s convention, rubber and amber rods are negatively charged while the glass rod is positively charged. If the net charge is zero in the object, it is said to be electrically neutral.
Following the pioneering
work of J. J. Thomson and E. Rutherford, in the late 19th century and in the
beginning of 20th century, we now understand that the atom is electrically
neutral and is made up of the negatively charged electrons, positively charged
protons, and neutrons which have zero charge. The material objects made up of atoms
are neutral in general. When an object is rubbed with another object (for
example rubber with silk cloth), some amount of charge is transferred from one
object to another due to the friction between them and the object is then said
to be electrically charged. Charging the objects through rubbing is
called triboelectric charging.
2. Basic properties of charges
(i) Electric charge
Most objects in the
universe are made up of atoms, which in turn are made up of protons, neutrons
and electrons. These particles have mass, an inherent property of particles.
Similarly, the electric charge is another intrinsic and fundamental property of
particles. The nature of charges is understood through various experiments
performed in the 19th and 20th century. The SI unit of charge is coulomb.
(ii) Conservation of charges
Benjamin Franklin argued
that when one object is rubbed with another object, charges get transferred
from one to the other. Before rubbing, both objects are electrically neutral
and rubbing simply transfers the charges from one object to the other. (For
example, when a glass rod is rubbed against silk cloth, some negative charge
are transferred from glass to silk. As a result, the glass rod is positively
charged and silk cloth becomes negatively charged). From these observations, he
concluded that charges are neither created or nor destroyed but can only be
transferred from one object to other. This is called conservation of total
charges and is one of the fundamental conservation laws in physics. It is stated
more generally in the following way.
‘The total electric
charge in the universe is constant and charge can neither be created nor
be destroyed. In any physical process, the net change in charge will always be
zero.
(iii) Quantisation of
charges
What is the smallest
amount of charge that can be found in nature? Experiments show that the charge
on an electron is −e and the charge on the proton is +e. Here, e
denotes the fundamental unit of charge. The charge q on any object is
equal to an integral multiple of this fundamental unit of charge e.

q = ne (1.1)
Here n is any
integer (0,±1,±2, ±3,
±4………..). This is called quantisation of electric charge. Robert Millikan in
his famous experiment found that the value of e = 1.6 × 10-19C. The charge
of an electron is −1.6 ×
10-19 C and the charge of the proton is +1.6 × 10-19C.
When a glass rod is
rubbed with silk cloth, the number of charges transferred is usually very
large, typically of the order of 1010. So the charge quantisation is not
appreciable at the macroscopic level. Hence the charges are treated to be
continuous (not discrete). But at the microscopic level, quantisation of charge
plays a vital role.
EXAMPLE 1.1
Calculate the number of
electrons in one coulomb of negative charge.
Solution
According to the
quantisation of charge
q = ne
Here q = 1C. So the
number of electrons in 1 coulomb of charge is
n = q/e = IC/1.6x10-10 = 6.25x1018 electrons
Related Topics