Chapter: 11th Physics : UNIT 8 : Heat and Thermodynamics
Calorimetry
Calorimetry
Calorimetry
means the measurement of the amount of heat released or absorbed by
thermodynamic system during the heating process. When a body at higher
temperature is brought in contact with another body at lower temperature, the
heat lost by the hot body is equal to the heat gained by the cold body. No heat
is allowed to escape to the surroundings. It can be mathematically expressed as
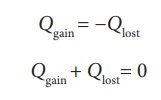
Heat
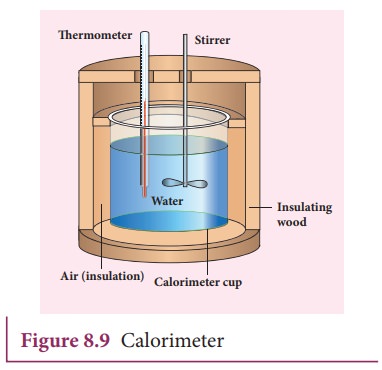
gained or lost is measured with a calorimeter. Usually the calorimeter is an
insulated container of water as shown in Figure 8.9.
A
sample is heated at high temperature (T1)
and immersed into water at room temperature (T2) in the calorimeter. After some time both sample and
water reach a final equilibrium temperature Tf
. Since the calorimeter is insulated, heat given by the hot sample is equal to
heat gained by the water. It is shown in the Figure 8.10
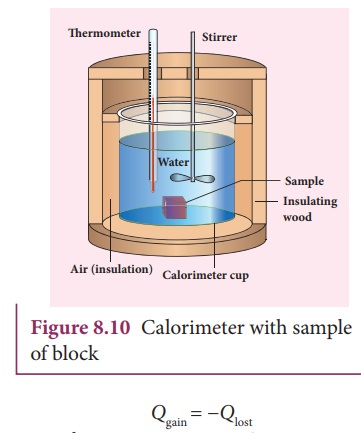
Note
the sign convention. The heat lost is denoted by negative sign and heat gained
is denoted as positive.
From
the definition of specific heat capacity
Qgain
=m2s2 (Tf ŌĆō
T2)
Qlost=
m1s1 (Tf ŌĆō
T1)
Here
s1 and s2 specific heat capacity of
hot sample and water respectively.
So
we can write
m2s2 (Tf ŌĆō
T2) = ŌłÆ m1s1 (Tf ŌĆō
T1)
m2s2Tf ŌĆō m2s2T2= ŌłÆ m1s1Tf + m1s1T1
m2s2Tf + m1s1Tf = m2s2T2 + m1s1T1
The
final temperature
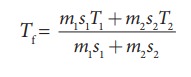
EXAMPLE 8.7
If 5 L of water at 50┬░C is mixed with 4L of water at 30┬░C, what will be the final temperature of water? Take the specific heat capacity of water as 4184 J kg-1K-1.
Solution
We
can use the equation
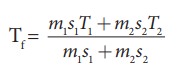
m1
= 5L = 5kg and m2= 4L = 4kg, s1 = s2
and T1=50┬░C =323K and T2 = 30┬░C=303 K.
So
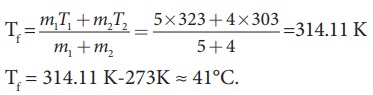
Tf
= 314.11 K-273K Ōēł 41┬░C.
Suppose
if we mix equal amount of water (m1 = m2) with 50┬░C and
30┬░C, then the final temperature is average of two temperatures.
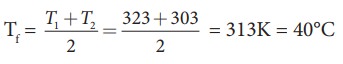
Suppose
if both the water are at 30┬░C then the final temperature will also 30┬░C. It
implies that they are at equilibrium and no heat exchange takes place between
each other.
Related Topics