Physics - Solved Example Problems for Propagation of Sound Waves | 11th Physics : UNIT 11 : Waves
Chapter: 11th Physics : UNIT 11 : Waves
Solved Example Problems for Propagation of Sound Waves
EXAMPLE 11.9
The ratio of the densities of oxygen and nitrogen is 16:14. Calculate the temperature when the speed of sound in nitrogen gas at 17°C is equal to the speed of sound in oxygen gas.
Solution
From equation (11.25), we have
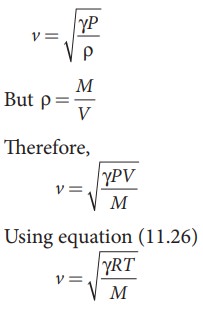
Where, R is the universal gas constant and M is the molecular mass of the gas. The speed of sound in nitrogen gas at 17°C is
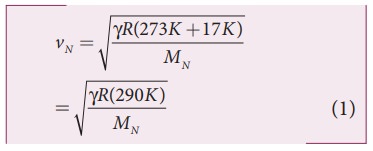
Similarly, the speed of sound in oxygen gas at t in K is
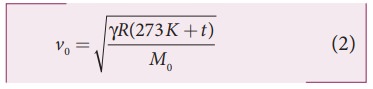
Given that the value of γ is same for both the gases, the two speeds must be equal. Hence, equating equation (1) and (2), we get
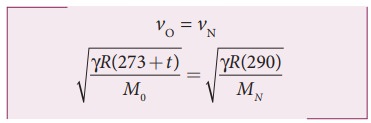
Squaring on both sides and cancelling γ R term and rearranging, we get
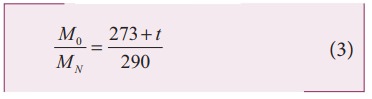
Since the densities of oxygen and nitrogen is 16:14,
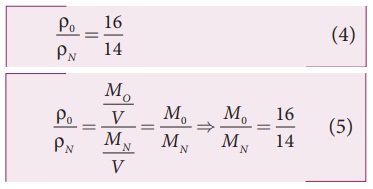
Substituting equation (5) in equation (3), we get

Related Topics