Chapter: 11th Physics : UNIT 7 : Properties of Matter
Microscopic Understanding of Various States of Matter
MICROSCOPIC
UNDERSTANDING OF VARIOUS STATES OF MATTER
Even
though various forms of matter such as solid food, liquids like water, and the
air that we breathe are familiar in the day – to – day lifestyle for the past
several thousand years, the microscopic understanding of solids, liquids, and
gases was established only in the 20th century. In the universe, everything is
made up of atoms. If so, why the same materials exist in three states? For
example, water exists in three forms as solid ice, liquid water, and gaseous
steam. Interestingly ice, water, and steam are made up of same types of
molecules; two hydrogen atoms and one oxygen atom form a water molecule.
Physics helps us to explore this beauty of nature at the microscopic level. The
distance between the atoms or molecules determines whether it exist in the
solid, liquid or gaseous state.
![]()
![]()
Solids
In
solids, atoms or molecules are tightly fixed. In the solid formation, atoms get
bound together through various types of bonding. Due to the interaction between
the atoms, they position themselves at a particular interatomic distance. This
position of atoms in this bound condition is called their mean positions.
Liquids
When
the solid is not given any external energy such as heat, it will remain as a
solid due to the bonding between atoms. When heated, atoms of the solid receive
thermal energy and vibrate about their mean positions. When the solid is heated
above its melting point, the heat energy will break the bonding between atoms
and eventually the atoms receive enough energy and wander around. Here also the
intermolecular (or interatomic) forces are important, but the molecules will
have enough energy to move around, which makes the structure mobile.
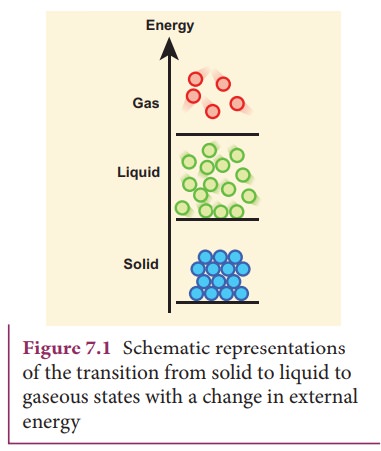
Gases
When
a liquid is heated at constant pressure to its boiling point or when the
pressure is reduced at a constant temperature it will convert to a gas. This
process of a liquid changing to a gas is called evaporation. The gas molecules
have either very weak bonds or no bonds at all. This enables them to move
freely and quickly. Hence, the gas will conform to the shape of its container
and also will expand to fill the container. The transition from solid to liquid
to gaseous states with the variation in external energy is schematically shown
in Figure 7.1.
In
the study of Newtonian mechanics (Volume 1), we assumed the objects to be
either as point masses or perfect rigid bodies (collection of point masses).
Both these are idealized models. In rigid bodies, changes in the shape of the
bodies are so small that they are neglected. In real materials, when a force is
applied on the objects, there could be some deformations due to the applied
force. It is very important to know how materials behave when a deforming force
is applied.
Related Topics