Chapter: 11th Physics : UNIT 7 : Properties of Matter
Fluids: Introduction
FLUIDS
Introduction
Fluids
are found everywhere in the world. Earth has about two-thirds of water and
one-third of land. Fluids are different from solids. Unlike solid, fluid has no
defined shape of its own. As far as fluid is concerned, liquid has fixed volume
whereas gas fills the entire volume of the container.
Pressure of a fluid:
Fluid
is a substance which begins to flow when an external force is applied on it. It
offers a very small resistance to the applied force. If the force acts on a
smaller area, then the impact will be more and vice versa. This particular idea
decides yet another quantity called ŌĆśpressureŌĆÖ.
Assume that an object is submerged in a fluid (say water) at rest. In this
case, the fluid will exert a force on the surface of the object. This force is
always normal to the surface of the object. If F is the magnitude of the normal
force acting on the surface area A, then the pressure is defined as the ŌĆśforce acting per unit areaŌĆÖ.

Pressure
is a scalar quantity. ItŌĆÖs S.I. unit and dimensions are NŌĆåm-2 or
pascal (Pa) and [ML-1T-2], respectively. Another common
unit of pressure is atmosphere, which is abbreviated as ŌĆśatmŌĆÖ. It is defined as
the pressure exerted by the atmosphere at sea level. i.e., 1 atm = 1.013 x 105
Pa or NŌĆåm-2. Apart from pressure, there are two more parameters such
as density and relative density (or specific gravity) which are used to
describe the nature of fluids.
Density of a fluid:
The
density of a fluid is defined as its mass per unit volume. For a fluid of mass
ŌĆśmŌĆÖ occupying volume ŌĆśVŌĆÖ, the
density Žü = m/V. The
dimensions and S.I unit of Žü are [ML-3] and kgŌĆåm-3,respectively.
It is a positive scalar quantity. Mostly, a liquid is largely incompressible
and hence its density is nearly constant at ambient pressure (i.e. at 1 atm.
pressure). In the case of gases, there are variations in densities with
reference to pressure.
Relative density or specific gravity:
The relative density of a substance is defined as the ratio of the density of a substance to the density of water at 4┬║C. It is a dimensionless positive scalar quantity. For example, the density of mercury is 13.6 ├Ś 103kgŌĆåm-3. Its relative density is equal to13.6x103kgm-3 / 1.0x103kgm-3 = 13.6.
EXAMPLE 7.6
A
solid sphere has a radius of 1.5 cm and a mass of 0.038 kg. Calculate the
specific gravity or relative density of the sphere.
Solution
Radius
of the sphere R = 1.5 cm
mass
m = 0.038 kg
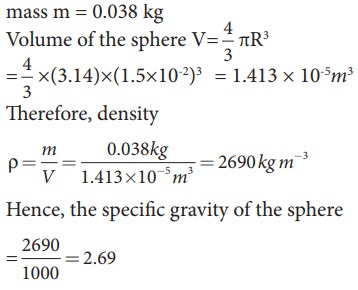
Related Topics