Chapter: Modern Analytical Chemistry: Electrochemical Methods of Analysis
Voltammetric Measurements
Voltammetric Measurements
Although early voltammetric methods relied on the use of only two
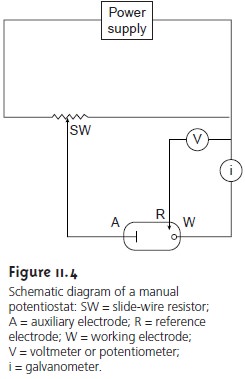
electrodes, modern voltammetry makes use of a three-electrode po-tentiostat, such as that shown
in Figure 11.4.
A time-dependent po- tential excitation signal is applied to the working
electrode, chang- ing its potential
relative to the fixed potential of the reference
electrode. The resulting
current between the working and auxiliary
electrodes is measured. The auxiliary electrode is generally a plat- inum wire,
and the SCE
and Ag/AgCl electrode are common refer- ence electrodes.
Several
different materials have been used as working elec-
trodes, including mercury,
platinum, gold, silver,
and carbon. The earliest voltammetric techniques, including
polarography, used mercury for the working electrode. Since
mercury is a liquid, the working electrode often consists of a
drop suspended from
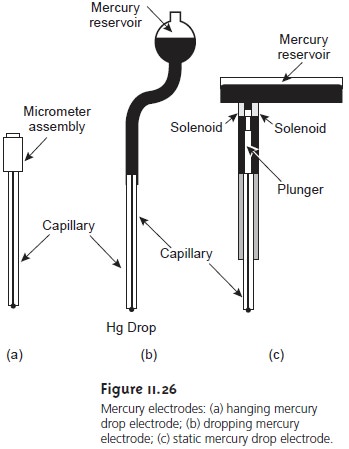
the end of a capillary tube (Figure 11.26).
In the hanging
mercury drop electrode, or HMDE,
a drop of the desired
size is formed
by the ac- tion of a micrometer screw
that pushes the
mercury through a narrow capillary tube. In the dropping mercury
electrode, or DME,
mercury drops form
at the end of
the capillary tube
as a result of gravity. Unlike the HMDE,
the mercury drop
of a DME grows
continuously and has
a finite lifetime of several seconds. At the end of
its lifetime the mercury drop is dislodged, either manually or by gravity,
and replaced by a new drop. The static mercury drop electrode,
or SMDE, uses a
solenoid-driven plunger to control the
flow of mercury. The SMDE can
be used as either a hanging mercury
drop electrode or as a dropping mercury
electrode. A single activation of the solenoid
momentarily lifts the plunger, allowing
enough mercury to flow
through the capillary to form a single drop.
To obtain a dropping mercury
elec- trode the solenoid
is activated repeatedly. A mercury film electrode consists
of a thin layer of mercury
deposited on the surface of a solid
carbon, platinum, or gold elec- trode. The solid electrode is placed in a solution
of Hg2+
and held at a potential at which the reduction of Hg2+ to Hg is favorable, forming a thin
mercury film.
Mercury has several
advantages as a working electrode. Perhaps its most im-
portant advantage is its high
overpotential for the
reduction of H3O+
to H2, which allows for the application of potentials as negative as 1 V versus the SCE in acidic
solutions, and –2 V versus
the SCE in basic solutions. A species such
as Zn2+, which is difficult to reduce at other electrodes without simultaneously reducing
H3O+, is easily reduced
at a mercury working electrode. Other advantages include
the ability of metals
to dissolve in the mercury, resulting in the
formation of an amalgam,
and the ability to easily renew the surface
of the electrode by extruding
a new drop. One limitation to its use as a working electrode is the ease with which
Hg is oxidized. For this reason,
mercury electrodes cannot
be used as at potentials more positive than –0.3 V to +0.4 V versus
the SCE, depending on the composition of the solution.
Solid electrodes constructed using platinum, gold,
silver, or carbon
may be used over a range of potentials, including potentials that are negative
and positive with respect
to the SCE. For example,
the potential range
for a Pt electrode extends from approximately +1.2 V to –0.2
V versus the
SCE in acidic
solutions and from +0.7 V to 1 V versus the SCE in basic solutions. Solid electrodes, therefore, can be used in place of mercury for many voltammetric analyses requiring negative
po- tentials and for voltammetric analyses
at positive potentials at which mercury
elec- trodes cannot be used. Except
for the carbon
paste electrode, solid
electrodes are fashioned into
disks that are
sealed into the
end of an inert support
and are in con-
tact with an electrical lead
(Figure 11.27). The
carbon paste electrode is made by filling the cavity at the end of the inert support
with a paste consisting of carbon
particles and a viscous oil. Solid electrodes are not without
problems, the most im-
portant of which is the
ease with which
the electrode’s surface
may be altered
by the adsorption of solution species
or the formation of oxide
layers. For this reason
solid electrodes need
frequent reconditioning, either
by applying an appropriate
potential or by polishing.
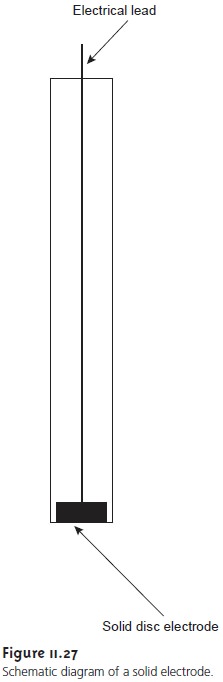
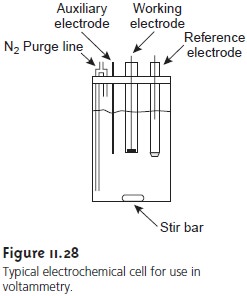
A typical arrangement for a voltammetric electrochemical cell is shown in Figure 11.28. Besides the working,
reference, and auxiliary
electrodes, the cell also in- cludes a N2 purge
line for removing
dissolved O2 and an optional
stir bar. Electro- chemical cells are available in a variety
of sizes, allowing
for the analysis
of solution volumes ranging
from more than 100 mL to as small as 50 μL.
Related Topics