Chapter: Modern Analytical Chemistry: Electrochemical Methods of Analysis
Current in Voltammetry
Current in Voltammetry
When
an analyte
is oxidized at the working
electrode, a current
passes electrons through
the external electric
circuitry to the auxiliary electrode, where reduc- tion of the
solvent or other components
of the solution matrix occurs. Reducing
an analyte at the
working electrode requires a source
of electrons, generating
a current that flows from the auxiliary
electrode to the cathode. In either case, a
current resulting from redox reactions at the working and auxiliary
electrodes is called
a faradaic current. In this section
we consider the factors
affecting the magnitude of this faradaic
current, as well as the
source of any nonfaradaic
currents.
Sign Conventions
Since the reaction of interest occurs
at the working
electrode, the classification of current is based on this reaction. A current due to the analyte’s
reduction is called a cathodic current
and, by convention, is considered positive. Anodic currents are
due to oxidation reactions and carry
a negative value.
Influence of Applied Potential on the Faradaic Current
As an example, let’s
con- sider the faradaic
current when a solution of Fe(CN) 63– is reduced
to Fe(CN) 6
4– at
|
6 |
|
6 |
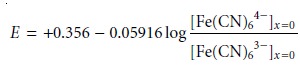
where +0.356 is the standard-state potential for the Fe(CN)63–/Fe(CN)64– redox couple, and
x
= 0 indicates that the concentrations of Fe(CN)63– and Fe(CN)64– are
those at the surface of the electrode. Surface concentrations are
used instead of bulk
concentrations since the equilibrium position
for the redox
reaction
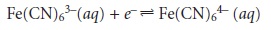
can only be established electrochemically at the electrode’s
surface.
Let’s assume that we have a solution
for which the concentration of Fe(CN)63– is
1.0 mM and
in which Fe(CN) 64– is absent.
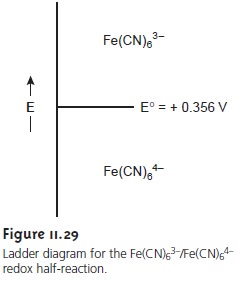
A ladder diagram
for this redox
example is shown
in Figure 11.29.
If a potential of +0.530
V is applied to the working
electrode, the concentrations of Fe(CN)63– and Fe(CN)64– at the surface of the elec- trode are unaffected, and no faradaic
current is observed. Switching the potential to +0.356 V, however,
requires that
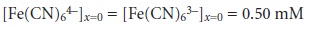
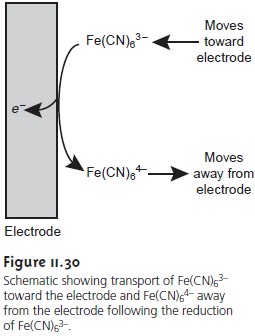
Which is only possible if a portion of the Fe(CN)63– at the electrode surface is re- duced to Fe(CN)64–. If this was all that occurred after the potential was applied, the result would be a brief surge of faradaic current that would quickly return to zero. However, although the concentration of Fe(CN)64– at the electrode surface is 0.50 mM, its concentration in the bulk of solution is zero. As a result, a concentration gradient exists between the solution at the electrode surface and the bulk solution. This concentration gradient creates a driving force that transports Fe(CN)64– away from the electrode surface (Figure 11.30). The subsequent decrease in the concen- tration of Fe(CN)64– at the electrode surface requires the further reductionof E
|
6 |
Although the applied
potential at the
working electrode determines if a faradaic current flows, the magnitude of the current
is determined by the rate of the result-
ing oxidation or reduction reaction
at the electrode surface. Two factors contribute to the rate of the electrochemical reaction: the rate
at which the
reactants and prod- ucts are transported to and from the surface
of the electrode, and the rate at which
electrons pass between
the electrode and
the reactants and
products in solution.
Influence of Mass Transport on the Faradaic Current
There are three modes of mass transport that influence the
rate at which
reactants and products are trans- ported to and from the electrode surface: diffusion, migration, and convection. Dif-
fusion from a region of high concentration to a region
of low concentration occurs
whenever the concentration of an ion or molecule at the surface
of the electrode is
different from that in bulk solution. When the potential applied to the working
electrode is sufficient to reduce
or oxidize the
analyte at the
electrode surface, a con-
centration gradient similar
to that shown
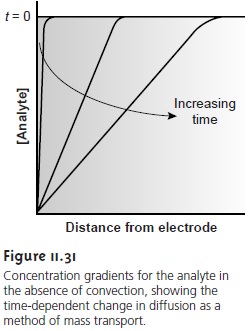
in Figure 11.31
is established. The
volume of solution in which the concentration gradient
exists is called
the diffusion layer. Without other modes of mass transport, the width of the diffusion
layer, δ, in- creases with time as the concentration of reactants near the electrode surface de- creases. The
contribution of diffusion to the rate
of mass transport, therefore, is
time-dependent.
Convection occurs when a mechanical means is used to carry reactants toward the electrode and to remove products
from the electrode. The most common
means of convection is to stir the solution
using a stir bar. Other methods include
rotating the electrode and incorporating the electrode into a flow cell.
The final mode of mass transport is migration,
which occurs when charged
particles in solution are attracted or repelled from an electrode that has a positive or negative surface charge. Thus, when the electrode is positively charged,
negatively charged particles move toward the electrode, while
positively charged particles move toward the bulk solution. Unlike
diffusion and convection, migration only af- fects the mass transport of charged particles.
The flux of material to and from
the electrode surface
is a complex function of all
three modes of mass transport. In the limit
in which diffusion is the only
signifi- cant means for the mass transport of the reactants
and products, the current in a
voltammetric cell is given by
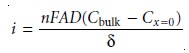 11.33
11.33
where n is the number
of electrons transferred in the redox reaction, F is Faraday’s constant, A is
the area of the electrode, D is the diffusion coefficient for the reactant
or product, Cbulk and Cx=0 are the concentration of the analyte
in bulk solution
and at the electrode surface, and δ is the
thickness of the
diffusion layer.
For equation 11.33
to be valid, convection and migration must not interfere with the formation of a diffusion layer between the electrode and the bulk of solu- tion. Migration is eliminated by adding a high concentration of an inert
supporting electrolyte to the analytical solution. Ions of similar
charge are equally
attracted or repelled from the surface
of the electrode and, therefore, have an equal probability
of undergoing migration. The large excess of inert ions, however, ensures
that few reactant and product ions will move as a result of migration. Although
convection may be easily
eliminated by not physically agitating the solution, in some situations it is desirable either
to stir the
solution or to push the
solution through an electro-
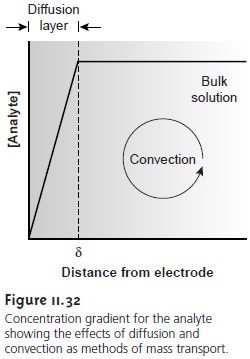
chemical flow cell. Fortunately, the dynamics of a fluid
moving past an electrode re- sults in a small
diffusion layer, typically of 0.001 – 0.01-cm thickness, in which the rate
of mass transport by convection drops
to zero (Figure
11.32).
Influence of the Kinetics of Electron Transfer on the Faradaic Current
The rate
of mass transport is one factor influencing the current in a voltammetric experiment. The ease with which electrons
are transferred between
the electrode and the reac- tants and products in solution also affects the current. When electron transfer
ki- netics are fast, the redox reaction is at equilibrium, and the concentrations of reac- tants and products at the electrode are those specified by the Nernst
equation. Such systems are considered electrochemically reversible. In other
systems, when electron transfer kinetics are sufficiently slow, the concentration of reactants and
products at the electrode surface, and thus the current,
differ from that predicted by the Nernst equation. In this case the system
is electrochemically irreversible.
Nonfaradaic Currents
Faradaic currents result from a redox
reaction at the elec-
trode surface. Other
currents may also exist in an electrochemical cell that are unre-
lated to any redox reaction.
These currents are called nonfaradaic currents
and must be accounted for if the
faradaic component of the measured current is to be
determined.
The most important example of a nonfaradaic current
occurs whenever the electrode’s potential is changed.
In discussing migration as a means
of mass trans- port, we noted that negatively charged
particles in solution
migrate toward a posi-
tively charged electrode, and positively charged particles move away from the same electrode. When an inert
electrolyte is responsible for migration, the result is a
structured electrode–surface interface called the electrical double
layer, or EDL, the
exact structure of which is of no concern in the context
of this text.
The movement of charged
particles in solution, however, gives rise to a short-lived, nonfaradaic charging current. Changing the potential of an electrode
causes a change in the structure of the EDL, producing a small charging
current.
Residual Current
Even in the absence of analyte, a small current
inevitably flows through an electrochemical cell.
This current, which
is called the residual
current, consists of two components: a faradaic current
due to the oxidation or reduction of trace
impurities, and the charging current.
Related Topics