Chapter: Modern Analytical Chemistry: Electrochemical Methods of Analysis
Evaluation - Potentiometric Methods of Analysis
Evaluation
Scale of Operation
The working
range for most
ion-selective electrodes is from
a maximum
concentration of 0.1–1 M to a minimum concentration of 10–5 –
10–10 M. This broad working
range extends from major to ultratrace ana- lytes, and is significantly greater than many other analytical methods. For conven- tional ion-selective electrodes, macro-sized samples with minimum
volumes of 0.05–10 mL are necessary. Microelectrodes and specially designed analyzers, such as
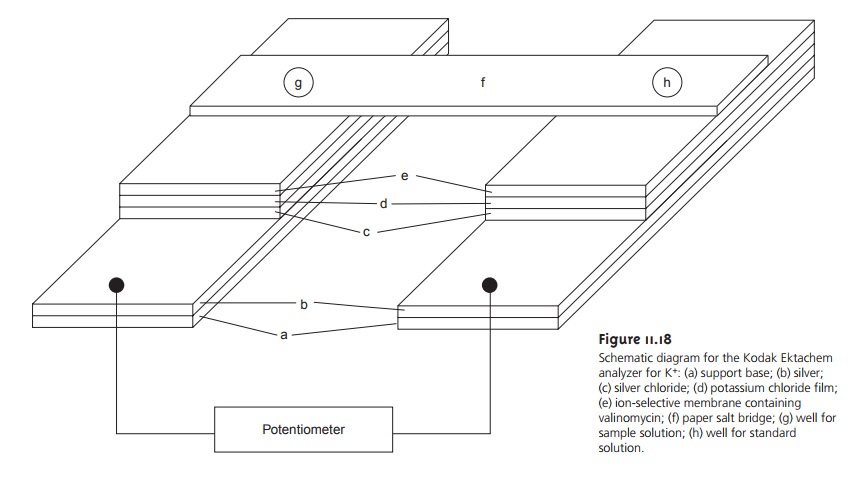
the Kodak Ektachem analyzer for K+ shown in Figure 11.18,
may be used
with ultramicro-sized samples
provided that the sample taken for analysis is suffi- ciently large to be representative of the original
sample.
Accuracy
The
accuracy of a potentiometric analysis
is limited by the measure- ment error for the
cell’s potential. Several
factors contribute to this measurement error, including the contribution to the potential from interfering ions,
the finite current drawn through
the cell while
measuring the potential, differences in the analyte’s activity coefficient in the sample and
standard solutions, and liquid
junction potentials. Errors
in accuracy due
to interfering ions
often can be elimi- nated by including a separation step
before the potentiometric analysis. Modern high-impedance potentiometers minimize errors due to the passage of current
through the electrochemical cell. Errors due to activity coefficients and liquid junction potentials are minimized
by matching the matrix of the standards to that of the sample.
Even in the
best circumstances, however, a difference in po- tential of approximately ±1 mV is observed for samples and standards at equal
concentration.
The effect of an uncertainty in potential on the accuracy of a potentiometric method of analysis is evaluated using
a propagation of uncertainty. For a membrane ion-selective electrode the general expression for potential is given as
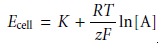
where z is the charge of the analyte.
From Table 4.9, the error
in the cell potential, ∆Ecell is
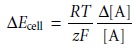
Rearranging and multiplying through by 100 gives the percent relative
error in con- centration as
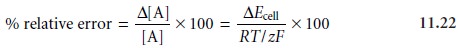
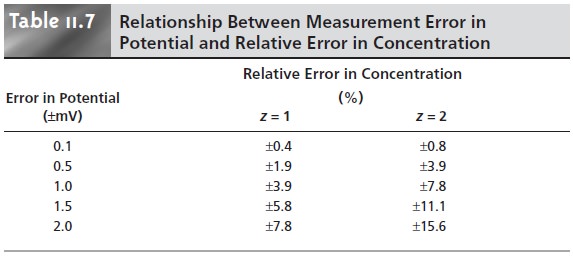
The relative measurement error in concentration, therefore, is
determined by the magnitude of the error in measuring the cell’s potential and by the charge of the an- alyte. Representative values are shown in Table 11.7 for ions with charges
of ±1 and ±2, at a temperature of 25 °C.
Accuracies of 1–5%
for monovalent ions
and 2–10% for divalent
ions are typical.
Although equation 11.22
was developed for membrane
electrodes, it also applies to metallic electrodes of the first
and second kind when z is replaced by n.
Precision
The precision of a
potentiometric measurement is limited by variations in temperature and the
sensitivity of the
potentiometer. Under most
conditions, and with simple, general-purpose potentiometers, the
potential can be measured with a repeatability of ±0.1 mV. From Table
11.7 this result
corresponds to an un-
certainty of ±0.4% for monovalent analytes, and ±0.8%
for divalent analytes. The reproducibility of potentiometric measurements is about
a factor of 10 poorer.
Sensitivity
The sensitivity of a potentiometric analysis is determined by the term RT/nF or RT/zF in the Nernst equation. Sensitivity is best for smaller values of n or z.
Selectivity
As described earlier, most ion-selective electrodes respond to more
than one analyte. For many ion-selective electrodes, however, the selectivity for the analyte is significantly greater
than for most interfering ions.
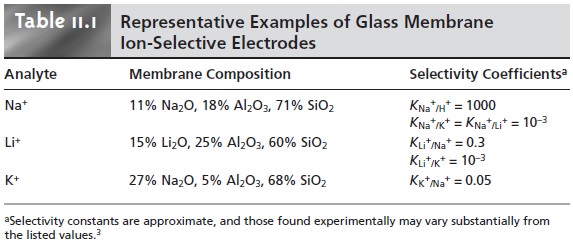
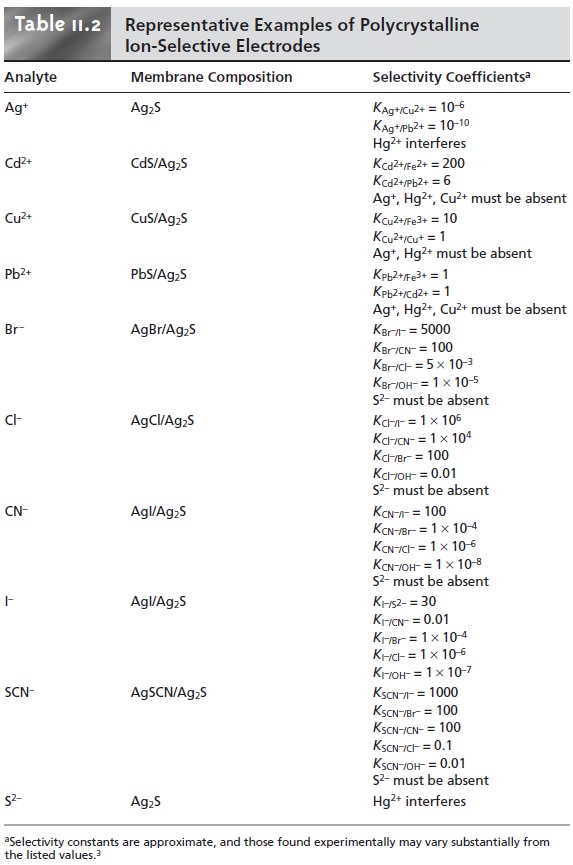
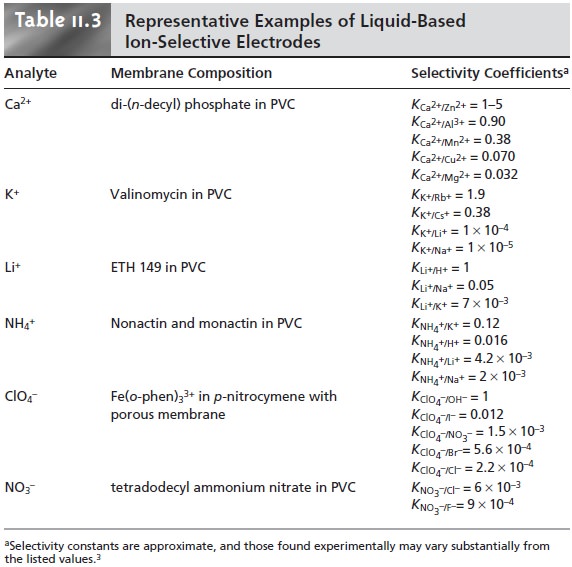
Published selectivity
coefficients for ion-selective electrodes (representative values are found in Tables 11.1 through
11.3) provide a useful guide
in helping the analyst determine whether a potentiometric analysis
is feasible for a given
sample.
Time, Cost, and Equipment
In comparison with
competing methods, potentiome- try provides a rapid, relatively low-cost means for analyzing samples.
Commercial instruments for measuring
pH or potential are available
in a variety of price ranges
and include portable models for use in the field.
Related Topics