Chapter: Modern Analytical Chemistry: Electrochemical Methods of Analysis
Selectivity of Membranes - Potentiometric Methods of Analysis
Selectivity of Membranes
Membrane potentials result
from a chemical interac-
tion between the analyte and active sites on the membrane’s surface.
Because the signal
depends on a chemical process, most membranes are
not selective toward
a single analyte.
Instead, the membrane
potential is proportional to the concen- tration of all ions in the sample solution capable of interacting at the mem- brane’s active sites. Equation
11.8 can be generalized to include the contribution of an interferent, I,
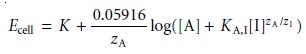
where zA and zI are the charges
of the analyte
and interferent, and
KA,I is a selectivity coefficient accounting for
the relative response of the interferent.* The selectivity coefficient is defined as
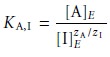
where [A]E and [I]E are the concentrations of analyte and
interferent yielding
identical cell potentials. When the selectivity coefficient is 1.00,
the membrane responds equally
to the analyte and interferent. A membrane shows
good se- lectivity for
the analyte when
KA,I is significantly less
than 1.00.
Selectivity coefficients for most commercially available ion-selective elec- trodes are provided by the manufacturer. If the selectivity coefficient is un- known, it can be determined
experimentally. The easiest method for determining KA,I is to prepare
a series of solutions, each of which
contains the same concentration of interferent, [I]add, but a different
concentration of analyte.
A plot of cell potential versus
the log of the analyte’s concentration has two dis-
tinct linear regions (Figure 11.11).
When the analyte’s concentration is signif- icantly larger than KA,I[I]add, the potential is a linear
function of log [A], as given by equation 11.8.
If KA,I[I]add
is significantly larger
than the analyte’s concentration, however,
the cell potential remains constant. The concentra-
tion of analyte and interferent at the intersection of these two linear regions
is used to calculate
KA,I.

Related Topics