Chapter: Modern Analytical Chemistry: Electrochemical Methods of Analysis
Coulometric Methods of Analysis
Coulometric Methods of Analysis
In potentiometry, the potential of an electrochemical cell under static
conditions is used to determine an analyte’s concentration. As seen in the preceding
section, po- tentiometry is an
important and frequently used quantitative method of analysis. Dynamic electrochemical methods, such as coulometry, voltammetry, and amper- ometry, in which current
passes through the electrochemical cell,
also are important analytical techniques. In this section
we consider coulometric methods of analysis.
Coulometric methods of analysis are based on an exhaustive electrolysis of the analyte. By exhaustive we mean that
the analyte is quantitatively oxidized or re- duced at the working
electrode or reacts quantitatively with a reagent
generated at the working
electrode. There are two forms of coulometry: controlled-potential coulometry, in which a constant potential is applied to the electrochemical cell, and
controlled-current coulometry, in which a constant current
is passed through
the electrochemical cell.
The total charge,
Q, in coulombs, passed during an electrolysis is related to the
absolute amount of analyte by Faraday’s
law
Q =
nFN ………………….. 11.23
where n is the number of electrons transferred per mole of analyte, F is
Faraday’s constant (96487 C mol–1), and
N
is the moles
of analyte. A coulomb is also equiva- lent to an A.s;
thus, for a constant current, i, the charge is given as
Q =
ite
………………….. 11.24
where te is the electrolysis time. If current
varies with time,
as it does in controlled- potential coulometry, then the total charge is given by
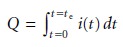 11.25
11.25
In coulometry, current and time are measured, and equation 11.24
or equation 11.25 is used to calculate Q. Equation 11.23 is then used to determine the moles of analyte. To obtain an accurate value for N, therefore, all the current
must result in the
analyte’s oxidation or reduction. In other words,
coulometry requires 100% current efficiency (or
an accurately measured
current efficiency established using a standard), a factor that
must be considered in designing a coulometric method
of analysis.
Related Topics