Chapter: Modern Analytical Chemistry: Electrochemical Methods of Analysis
Glass Ion-Selective Electrodes - Potentiometric Methods of Analysis
Glass Ion-Selective Electrodes
The first commercial glass electrodes were manu- factured
using Corning 015, a glass with a composition of approximately 22% Na2O, 6% CaO, and
72% SiO2. When immersed in an aqueous
solution, the outer approximately 10 nm of the membrane becomes hydrated over
the course of sev-
eral
hours. Hydration of the glass membrane results in the formation
of negatively charged sites, G–, that are part of the glass membrane’s silica
framework. Sodium ions,
which are able to move through the hydrated layer,
serve as the counterions. Hydrogen ions from solution diffuse
into the membrane
and, since they bind more strongly
to the glass than does Na+, displace
the sodium ions
H+(aq)+ G––Na+(s) < = = = = > G––H+(s)+ Na+(aq)
giving rise to the membrane’s selectivity for H+. The transport of charge across
the membrane is carried
by the Na+ ions. The potential of glass electrodes using Corn- ing 015 obeys the equation
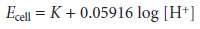 11.9
11.9
over a pH range of approximately 0.5–9.
Above a pH of 9–10,
the glass membrane may become more responsive to other cations, such as Na+ and K+.
Replacing Na2O and
CaO with Li2O and BaO extends
the useful pH range of glass
membrane electrodes to pH levels
greater than 12.
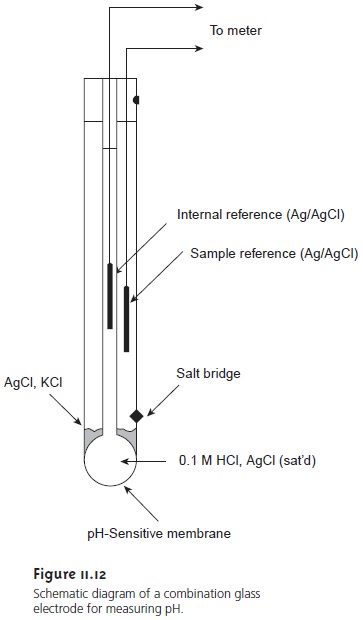
Glass membrane pH electrodes are often available in a combination form that includes both the indicator and the reference electrode. The use of a single electrode greatly simplifies the measurement of pH. An example of a typical
combination electrode is shown
in Figure 11.12.
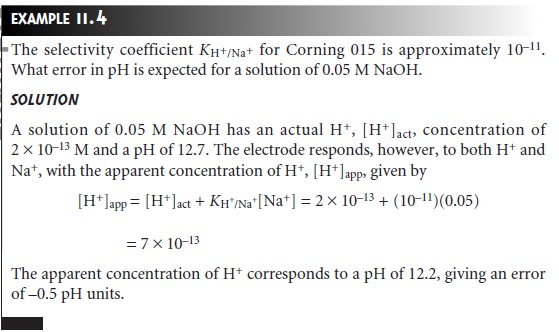
The response of the Corning
015 glass membrane
to monovalent cations
other than H+ at high pH led to the development of glass membranes
possessing a greater selectivity for other cations.
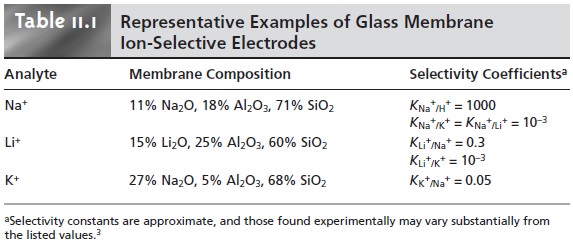
For example, a glass membrane
with a composition of 11% Na2O, 18% Al2O3, and 71% SiO2 is used as a Na+ ion-selective electrode. Other glass electrodes have been developed for the analysis
of Li+, K+, Rb+, Cs+, NH4+, Ag+,
and Tl+. Several
representative examples of glass membrane electrodes are
listed in Table 11.1.
Since the typical
thickness of the glass membrane
in an ion-selective electrode
is about 50 μm, they must be handled carefully to prevent the formation of cracks
or breakage. Before a glass electrode can be used it must be conditioned by soaking for several
hours in a solution containing the analyte. Glass
electrodes should not
be allowed to dry out, as this destroys
the membrane’s hydrated
layer. If a glass elec- trode has been allowed
to dry out,
it must be reconditioned before
it can be used.
The composition of a glass membrane changes over time, affecting the electrode’s performance. The
average lifetime for
a glass electrode is several years.
Related Topics