Chapter: Modern Analytical Chemistry: Electrochemical Methods of Analysis
Reference Electrodes - Potentiometric Methods of Analysis
Reference Electrodes
Potentiometric electrochemical cells are constructed such that one of the half-cells
provides a known reference potential, and the potential of the other
half-cell indi- cates the analyte’s concentration. By convention, the reference electrode is taken to be
the anode; thus,
the shorthand notation for a potentiometric electrochemical cell is
The ideal reference electrode must provide
a stable potential so that any change in Ecell is attributed to the indicator electrode, and, therefore, to a change
in the ana- lyte’s concentration. In addition, the ideal reference electrode should be easy to make
and to use. Three common
reference electrodes are discussed in this section.
Standard Hydrogen Electrode
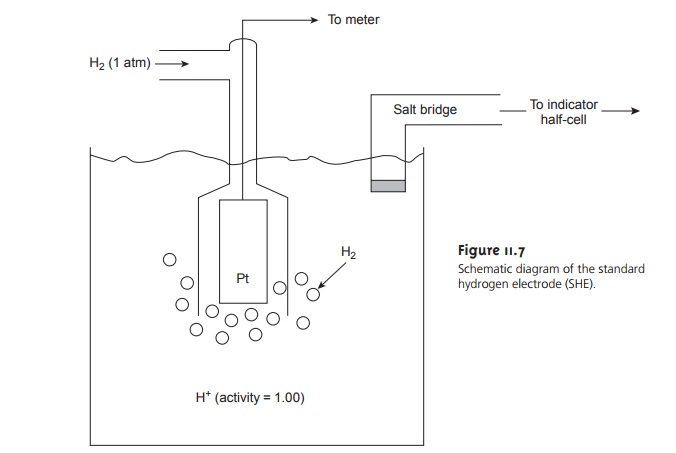
The standard hydrogen electrode (SHE) is rarely used for routine analytical work, but is important because it is the reference elec- trode used to establish standard-state potentials for other half-reactions. The SHE consists of a Pt electrode immersed in a solution in which the hydrogen ion activity is 1.00 and in which H2 gas is bubbled at a pressure of 1 atm (Figure 11.7). A con- ventional salt bridge connects the SHE to the indicator half-cell.
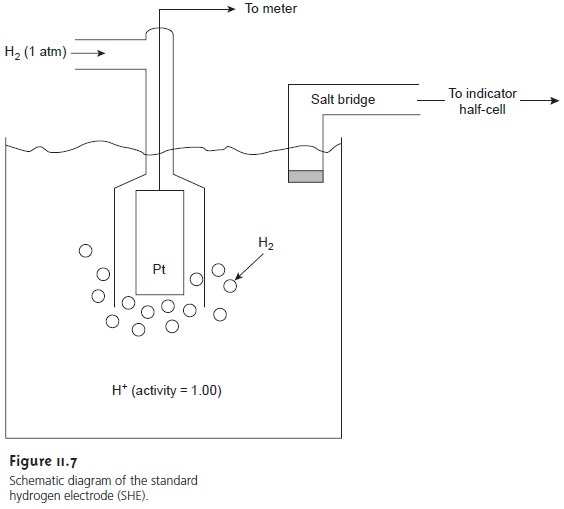
The shorthand
no- tation for the standard hydrogen
electrode is
Pt(s), H2 (g, 1
atm) | H+ (aq, a =
1.00) ||
and the standard-state potential for the reaction
2H+(aq)+ e– < = = = = > H2(g)
is, by definition, 0.00 V for all temperatures. Despite its importance as the funda- mental reference electrode against
which all other
potentials are measured, the SHE is rarely
used because it is difficult to prepare and inconvenient to use.
Calomel Electrodes
Calomel reference electrodes are based on the redox
couple between Hg2Cl2 and Hg (calomel
is a common name for Hg2Cl2).
Hg2Cl2(s) +2e– < = = = = > 2Hg(l)
+ 2Cl–(aq)
The Nernst equation for the calomel electrode is
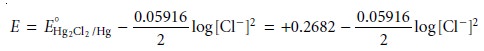
The potential of a calomel electrode, therefore, is determined
by the concentration of Cl–.
The saturated
calomel electrode (SCE), which is constructed using an aqueous solution saturated with KCl,
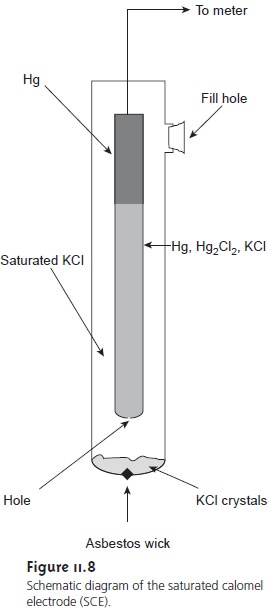
has a potential at 25 °C of +0.2444 V. A typical
SCE is shown in Figure 11.8 and consists
of an inner tube, packed
with a paste of Hg, Hg2Cl2, and saturated KCl, situated within a second tube filled with a saturated so- lution of KCl. A small hole connects the two tubes, and an asbestos fiber serves as a
salt bridge to the solution
in which the SCE is immersed. The stopper in the outer tube may be removed
when additional saturated
KCl is needed. The shorthand
no- tation for this cell is
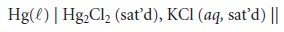
The SCE has the advantage
that the concentration of Cl–, and, therefore, the poten-
tial of the electrode, remains
constant even if the KCl solution partially
evaporates. On the other
hand, a significant disadvantage of the
SCE is that
the solubility of KCl
is sensitive to a change
in temperature. At higher temperatures the concentration of Cl– increases, and the electrode’s potential decreases. For example, the potential of the
SCE at 35 °C is +0.2376 V. Electrodes containing unsaturated solutions of KCl
have potentials that
are less temperature-dependent, but
experience a change
in po- tential if the concentration of KCl increases
due to evaporation. Another disadvan- tage to calomel electrodes is that they
cannot be used
at temperatures above
80 °C.
Silver/Silver Chloride Electrodes
Another
common
reference electrode is the silver/silver chloride
electrode, which is based on the redox
couple between AgCl and Ag.
AgCl(s)+ e– < = = = = > Ag(s)+
Cl–(aq)
As with the
saturated calomel electrode, the potential of the Ag/AgCl
electrode is determined by the concentration of Cl– used in its preparation.
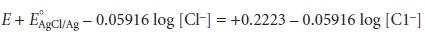
When prepared using
a saturated solution
of KCl, the Ag/AgCl electrode has a potential of +0.197 V at 25 °C. Another
common Ag/AgCl electrode
uses a so- lution of 3.5 M KCl and has a potential of +0.205 at 25 °C. The Ag/AgCl
elec- trode prepared with saturated KCl, of course, is more temperature-sensitive than one prepared with an unsaturated solution of KCl.
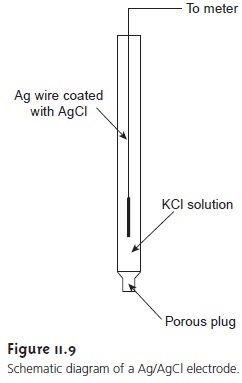
A typical Ag/AgCl
electrode is shown
in Figure 11.9 and consists
of a sil- ver wire, the
end of which
is coated with
a thin film
of AgCl. The
wire is im- mersed in a solution
that contains the desired concentration of KCl and that is saturated with AgCl. A porous plug serves as the salt bridge. The shorthand
notation for the cell is
Ag(s) | AgCl (sat’d), KCl (x M) ||
where x is the concentration of KCl.
In comparison to the SCE the Ag/AgCl
electrode has the advantage of being useful
at higher temperatures. On the other
hand, the Ag/AgCl
electrode is more prone
to reacting with
solutions to form
insoluble silver complexes that may plug the
salt bridge between
the electrode and
the solution.
Related Topics