Chapter: Modern Analytical Chemistry: Electrochemical Methods of Analysis
Crystalline Solid-State Ion-Selective Electrodes - Potentiometric Methods of Analysis
Crystalline Solid-State
Ion-Selective Electrodes
Solid-state ion-selective electrodes
use membranes fashioned from polycrystalline or single-crystal
inorganic salts. Polycrystalline ion-selective electrodes are made
by forming a thin pellet
of Ag2S, or a mixture of Ag2S and either a second silver
salt or another
metal sulfide. The pellet,
which is 1–2 mm in thickness, is sealed into the end of a nonconducting
plastic cylinder, and
an internal solution containing the analyte
and a reference electrode are placed in the cylinder. Charge is carried
across the membrane
by Ag+ ions.
The membrane potential for a Ag2S pellet develops as the result
of a difference in the equilibrium position of the solubility reaction
Ag2S(s) < = = = = > 2Ag+(aq)+ S2–(aq)
on the two
sides of the
membrane. When used
to monitor the
concentration of Ag+ ions, the cell potential
is
Ecell = K + 0.05916 log [Ag+]
The membrane also
responds to the
concentration of S2–,
with the cell
potential given as
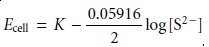
If a mixture
of an insoluble silver salt and Ag2S is used to make the membrane,
then the membrane potential also responds to the concentration of the anion
of the added silver
salt. Thus, pellets
made from a mixture of Ag2S and AgCl can serve as a
Cl– ion-selective electrode, with a cell
potential of
Ecell = K – 0.05916 log [Cl–]
Membranes fashioned from
a mixture of Ag2S with
CdS, CuS, or PbS are
used to make ion-selective electrodes that respond
to the concentration of Cd2+, Cu2+,
or Pb2+. In this case the cell potential is
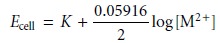
where [M2+] is the concentration of the appropriate metal ion.
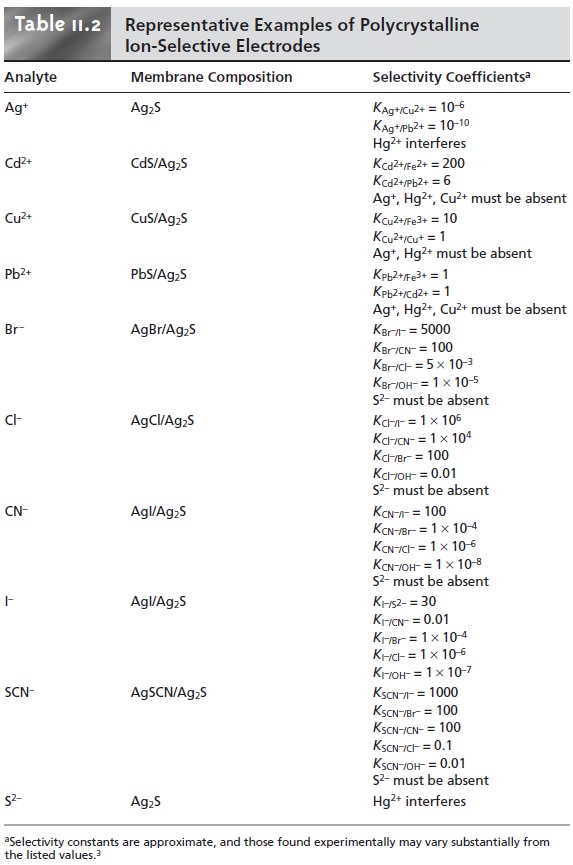
Several examples of polycrystalline, Ag2S-based
ion-selective electrodes are listed in Table 11.2.
The selectivity of these ion-selective electrodes is determined by solubility. Thus, a Cl– ion-selective electrode constructed using a Ag2S/AgCl mem- brane is more selective for Br– (KCl–/Br– =
102) and
I– (KCl–/l– =
106) since
AgBr and AgI are
less soluble than
AgCl. If the
concentration of Br– is sufficiently high, the AgCl at
the membrane–solution interface is replaced by AgBr, and the electrode’s response to Cl– decreases substantially. Most of the ion-selective electrodes listed in Table 11.2 can be used over
an extended range
of pH levels. The equilibrium be- tween S2– and HS– limits the
analysis for S2– to a pH range
of 13–14. Solutions of CN–, on the
other hand, must
be kept basic
to avoid the
release of HCN.
The membrane of a F– ion-selective electrode
is fashioned from a single crystal
of LaF3 that
is usually doped
with a small
amount of EuF2 to enhance the
mem- brane’s conductivity. Since
EuF2 provides only two F– ions, compared
with three for LaF3, each EuF2 produces a vacancy
in the crystal lattice. Fluoride ions move through the membrane by moving into adjacent vacancies. The LaF3 membrane is sealed into the end of a nonconducting plastic tube, with a standard
solution of F–, typically 0.1 M NaF, and a Ag/AgCl reference electrode.
The membrane
potential for a F– ion-selective electrode results from a difference in the solubility of LaF3 on opposite
sides of the membrane, with the potential
given by
Ecell = K – 0.05916 log [F–]
One advantage of the F– ion-selective electrode is its freedom
from interference. The only significant exception is OH– (KF–/OH– = 0.1), which imposes
a maximum pH limit
for a successful analysis.
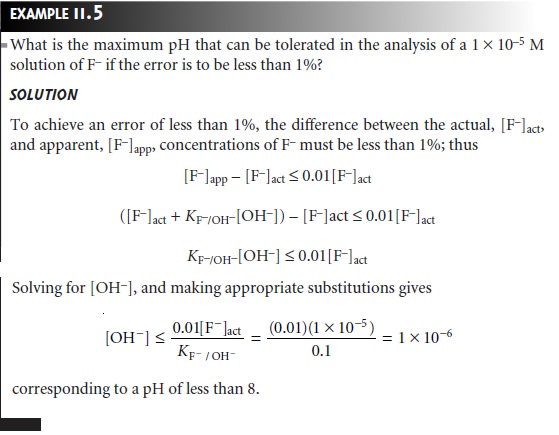
Below a pH of 4 the predominate form of fluoride in solution is HF, which,
unlike F–, does not contribute to the membrane
potential. For this reason, an analysis for total fluoride must be carried out at a pH greater
than 4.
Unlike ion-selective electrodes using glass membranes, crystalline solid-state
ion-selective electrodes do not need to be conditioned before
use and may be stored dry. The surface of the electrode is subject to poisoning, as described earlier
for a Cl– ISE in contact with an excessive concentration of Br–. When this happens, the electrode can be returned to its original condition by sanding
and polishing the crystalline membrane.
Related Topics