Chapter: Modern Analytical Chemistry: Electrochemical Methods of Analysis
Quantitative Applications - Coulometric Methods of Analysis
Quantitative Applications
Coulometry may be used for the quantitative analysis of both inorganic and organic
compounds. Examples of controlled-potential and controlled-current coulometric methods are discussed in the following sections.
Controlled-Potential Coulometry
The majority of controlled-potential coulometric analyses involve
the determination of inorganic cations
and anions, including trace metals and halides.
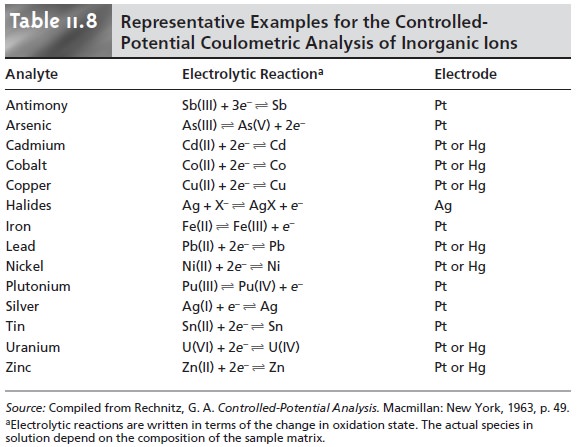
Table 11.8 provides
a summary of several of these methods.
The ability to control selectivity by carefully selecting the
working electrode’s potential, makes controlled-potential coulometry particularly useful for the analysis of alloys. For example, the composition of an alloy
containing Ag, Bi,
Cd, and Sb can
be determined by dissolving the sample and placing it in a matrix of 0.2 M H2SO4. A platinum working
electrode is immersed
in the solution and held at a constant potential of +0.40 V versus the SCE. At this potential Ag(I) deposits on the
Pt electrode as Ag, and the other metal ions remain in solution. When electrolysis is complete, the total charge
is used to determine the amount of silver in the alloy. The potential of the
platinum electrode is then shifted
to –0.08 V versus the
SCE, depositing Bi on the working
electrode. When the coulometric analysis
for bismuth is complete, antimony is determined by shifting the working electrode’s potential to –0.33 V versus the
SCE, depositing Sb.
Finally, cadmium is determined following its electrodeposition on the Pt electrode at a potential of –0.80 V versus the SCE.
Another area where controlled-potential coulometry has found
application is in nuclear chemistry, in which elements
such as uranium and polonium
can be determined at trace levels. For example, microgram quantities of uranium in a medium
of H2SO4 can be determined by reducing U(VI)
to U(IV) at a mercury working electrode.
Controlled-potential coulometry also can be applied to the quantitative analy- sis of organic
compounds, although the number of applications is significantly less than
that for inorganic analytes. One example
is the six-electron reduction of a nitro
group, –NO2, to a primary
amine, –NH2, at a mercury
electrode. Solutions of picric acid, for instance, can be analyzed by reducing to triaminophenol.
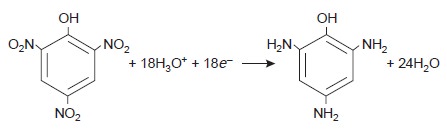
Another example is the successive reduction of trichloroacetate to dichloroac-
etate, and of dichloroacetate to monochloroacetate
Cl3CCOO–(aq)+ H3O+(aq)+ 2e– < =
= = = > Cl2HCCOO–(aq)+ Cl–(aq)+ H2O(l)
Cl2HCCOO–(aq)+ H3O+(aq)+ 2e– < =
= = = > ClH2CCOO–(aq)+ Cl–(aq)+ H2O(l)
Mixtures of trichloroacetate and dichloroacetate are analyzed by selecting an initial
potential at which only the more easily
reduced trichloroacetate is reduced. When its
electrolysis is complete,
the potential is switched to a more negative potential
at which dichloroacetate is reduced. The
total charge for
the first electrolysis is used to determine the amount of trichloroacetate, and the difference in total charge
be- tween the first
and second electrolyses gives the amount
of dichloroacetate.
Controlled-Current Coulometry
The
use of a mediator makes
controlled-current coulometry a more versatile analytical method than controlled-potential coulome- try. For example, the direct oxidation or reduction of a protein
at the working elec- trode in controlled-potential coulometry is difficult if the protein’s active redox site lies deep within its structure. The controlled-current coulometric analysis of the protein is made possible, however, by coupling
its oxidation or reduction to a medi- ator that is reduced
or oxidized at the working
electrode. Controlled-current coulo- metric methods have been developed for many of the same analytes that may be de-
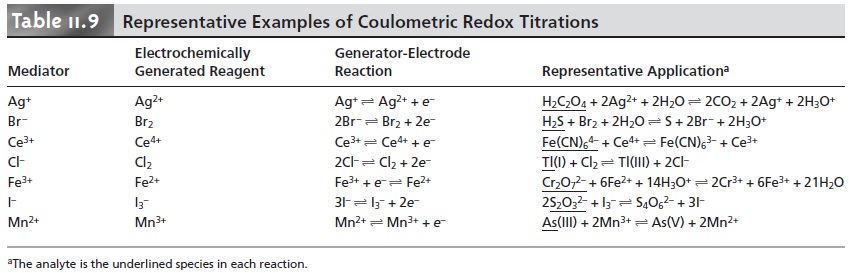
termined by conventional redox titrimetry. These methods, several
of which are summarized in Table 11.9,
also are called
coulometric redox titrations.
Coupling the mediator’s oxidation or reduction to an acid–base, precipitation, or complexation reaction
involving the analyte
allows for the coulometric titration of analytes that are not easily oxidized or reduced. For example, when using H2O as a mediator, oxidation at the anode
produces H3O+
6H2O(l) < = = = = > 4H3O+(aq)+ O2(g)+ 4e–
while reduction at the cathode produces OH–.
2H2O(l)+ 2e– < = = = = > 2OH–(aq)+ H2(g)
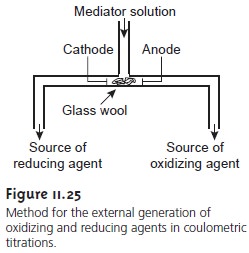
If the oxidation or reduction of H2O is carried out externally using the generator cell shown in Figure 11.25, then H3O+ or OH– can be dispensed selectively into a solution containing a basic or acidic analyte. The resulting reaction is identical to that in an acid–base titration. Coulometric acid–base titrations have been used for the analysis of strong and weak acids and bases, in both aqueous and nonaqueous matrices.
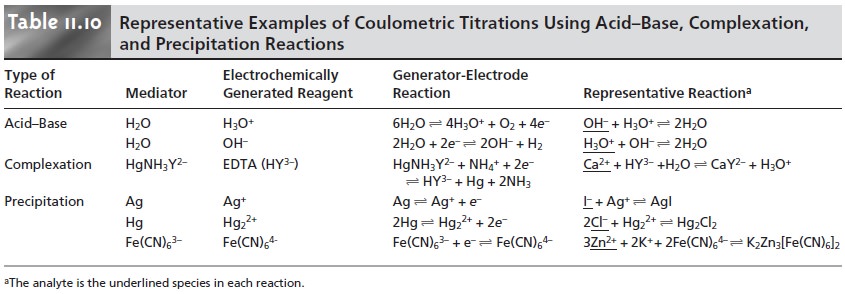
Examples of coulometric titrations involving acid–base, complexation,
and precipitation reactions are summarized in Table 11.10.

In comparison with
conventional titrimetry, there
are several advantages to the coulometric titrations listed in Tables
11.9 and 11.10.
One advantage is that the electrochemical generation of a “titrant” that reacts immediately with the analyte
al- lows the use of reagents
whose instability prevents
their preparation and storage as a
standard solution. Thus,
highly reactive reagents
such as Ag2+ and Mn3+ can be used
in coulometric titrations. Because it is relatively easy to measure
small quantities of charge, coulometric titrations can be used to determine
small quantities of analyte
that cannot be measured accurately by a conventional titration.
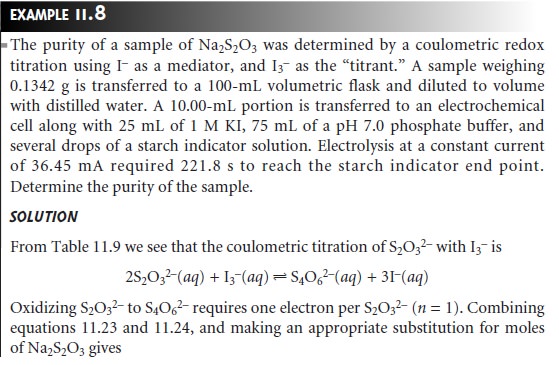
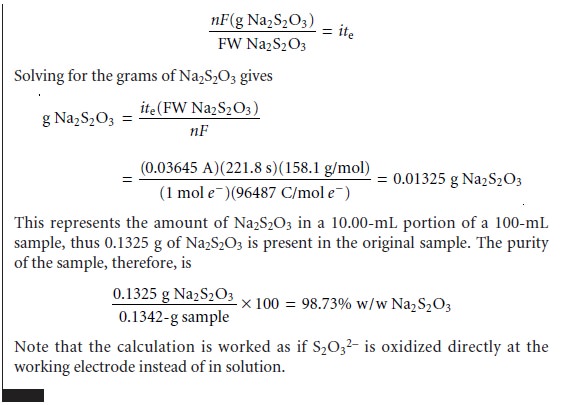
Quantitative Calculations
The absolute amount
of analyte in a coulometric analy- sis is determined by applying Faraday’s
law (equation 11.23) with the total charge during the electrolysis given
by equation 11.24
or equation 11.25.
Example 11.8 shows the calculations for a typical
coulometric analysis.
Representative Method
Every controlled-potential or controlled-current coulo- metric method has its own unique considerations. Nevertheless, the following pro- cedure for the determination of dichromate by a coulometric redox titration pro- vides an instructive example.
Related Topics