Home | | Modern Analytical Chemistry | Characterization Applications - Coulometric Methods of Analysis
Chapter: Modern Analytical Chemistry: Electrochemical Methods of Analysis
Characterization Applications - Coulometric Methods of Analysis
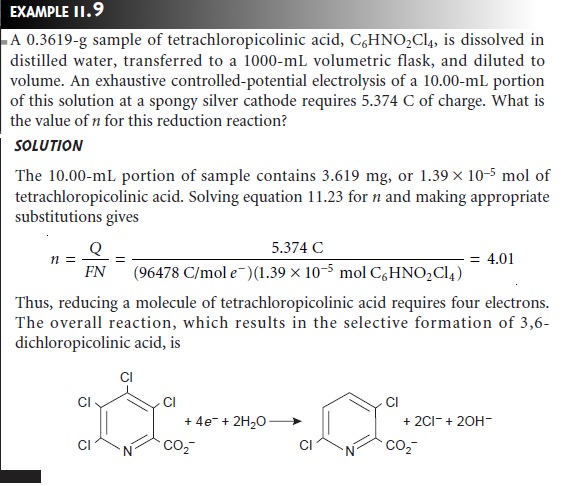
Studies aimed at characterizing the mechanisms of electrode reactions often make use of coulometry for determining the number of electrons involved in the reaction.
Characterization Applications
Studies aimed at characterizing the mechanisms of electrode reactions often make use of coulometry for determining the number of electrons involved
in the reaction. To make such
measurements a known
amount of a pure compound is subject to a
controlled-potential electrolysis. The coulombs of charge needed to complete
the electrolysis are used to determine
the value of n using Faraday’s
law (equation 11.23).
Study Material, Lecturing Notes, Assignment, Reference, Wiki description explanation, brief detail
Modern Analytical Chemistry: Electrochemical Methods of Analysis : Characterization Applications - Coulometric Methods of Analysis |
Related Topics
Modern Analytical Chemistry: Electrochemical Methods of Analysis