Chapter: Modern Analytical Chemistry: Electrochemical Methods of Analysis
Controlled-Potential Coulometry
Controlled-Potential Coulometry
The easiest method
for ensuring 100% current efficiency is to maintain
the working electrode at a constant
potential that allows
for the analyte’s quantitative oxidation
or reduction, without simultaneously oxidizing or reducing an interfering species. The current flowing through an
electrochemical cell under a constant potential is proportional to the analyte’s concentration. As electrolysis progresses the analyte’s
concentration decreases, as does the
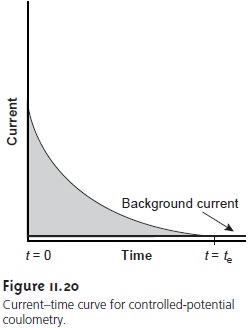
current. The resulting current-versus-time pro-
file
for controlled-potential coulometry, which also is known as potentiostatic
coulometry, is shown in Figure 11.20. Integrating the area under the curve (equa-
tion 11.25), from t = 0 until t =
te, gives
the total charge.
In this section
we consider the experimental parameters and instrumentation needed to develop
a controlled- potential coulometric method of analysis.
Selecting a Constant Potential
In controlled-potential coulometry, the potential is selected so that the desired oxidation
or reduction reaction
goes to completion without interference from redox reactions involving other components of the sam- ple
matrix. To see how an appropriate potential
for the working electrode is se-
lected, let’s develop a constant-potential coulometric method for Cu2+ based on its reduction to copper metal
at a Pt cathode working
electrode.
Cu2+(aq)+ 2e– < = = = = > Cu(s)
………………….. 11.26
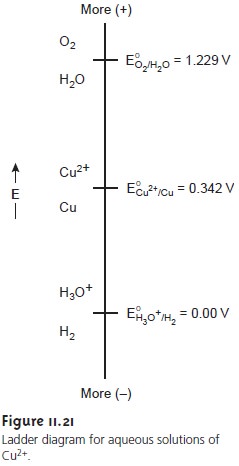
A ladder diagram
for a solution of Cu2+ (Figure 11.21)
provides a useful
means for evaluating the
solution’s redox properties. From the ladder
diagram we can
see that reaction 11.26 is
favored when the working electrode’s potential is more negative than +0.342
V versus the SHE (+0.093
V versus the SCE). To maintain a 100% cur- rent efficiency, however, the
potential must be selected so that the
reduction of H3O+ to H2 does not contribute significantly to the total
charge passed at the electrode.
The potential needed
for a quantitative reduction of Cu2+ can be calculated using the Nernst equation
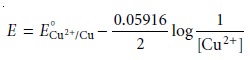 11.27
11.27

where [Cu2+]0
is the initial concentration of Cu2+ in the sample. Substituting equa- tion 11.28
into equation 11.27 gives the desired potential electrode as
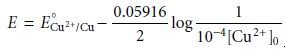
If the initial
concentration of Cu2+ is 1.00
x 10–4
M, for example, then the cathode’s potential must be more negative than +0.105 V versus the SHE (–0.139
V versus the SCE)
to achieve a quantitative reduction of Cu2+ to Cu. Note that
at this potential H3O+ is not reduced to H2, maintaining a 100% current
efficiency. Many of the
published procedures for the controlled-potential coulometric analysis of Cu2+ call
for potentials that are more negative than that shown
for the reduction of H3O+ in Figure 11.21.12 Such potentials can be used,
however, because the
slow kinetics for reducing H3O+ results in a significant overpotential that shifts the potential of the H3O+/H2 redox
couple to more
negative potentials.
Minimizing Electrolysis Time
The current-time curve for controlled-potential coulometry in
Figure 11.20 shows that the current decreases continuously
throughout electrolysis. An exhaustive electrolysis, therefore, may
require a long time. Since time is an important consideration in choosing and designing analyt- ical methods, the factors
that determine the analysis time need to be considered.
The change in current as a function
of time in controlled-potential coulometry is approximated by an exponential decay;
thus, the current
at time t is
i =
i0e–kt
………………….. 11.29
where i0 is the initial
current, and k is
a constant that is directly
proportional to the area
of the working electrode and the rate of stirring
and inversely proportional to the volume of the solution. For an exhaustive electrolysis in which
99.99% of the analyte is oxidized or reduced, the
current at the
end of the
analysis, te, may be ap- proximated as
i (10–4)i0 ………………….. 11.30
Substituting equation 11.30
into equation 11.29
and solving for te gives the mini- mum time for an exhaustive electrolysis as
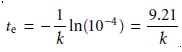
From this equation we see that
increasing k leads to a shorter
analysis time. For
this reason controlled-potential coulometry is carried out
in small-volume electrochem- ical cells, using electrodes with large surface
areas and with high stirring
rates. A quantitative electrolysis typically requires approximately 30–60 min, although
shorter or longer
times are possible.
Instrumentation
The potential in controlled-potential coulometry is set using
a three-electrode potentiostat. Two types of working electrodes are commonly
used: a Pt electrode manufactured from platinum-gauze and
fashioned into a cylindrical tube, and an Hg pool electrode. The large overpotential for reducing H3O+ at
mercury makes it the electrode of choice for
analytes requiring negative potentials. For
example, potentials more
negative than –1 V versus
the SCE are feasible at an Hg electrode (but not at a Pt electrode), even in very acidic solu- tions. The ease with which mercury
is oxidized, however,
prevents its use at po- tentials that are positive
with respect to the SHE. Platinum working
electrodes are used when positive potentials are required. The auxiliary electrode, which is often a Pt wire,
is separated by a salt
bridge from the
solution containing the
an- alyte. This is necessary to prevent electrolysis products generated at the auxiliary electrode from
reacting with the
analyte and interfering in the analysis. A satu- rated calomel
or Ag/AgCl electrode serves as the reference electrode.
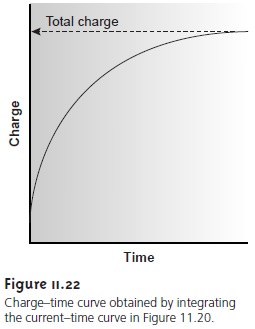
The other essential feature of instrumentation for controlled-potential coulom- etry is a means
of determining the total charge
passed during electrolysis. One method is to monitor
the current as a function
of time and determine the area
under the curve (see Figure 11.20). Modern instruments, however, use electronic
integration to monitor
charge as a function of time. The
total charge at the end
of the electrolysis then can be read directly from a digital readout or
from a plot of charge versus time (Figure
11.22).
Related Topics