Chapter: Modern Analytical Chemistry: Electrochemical Methods of Analysis
Metallic Indicator Electrodes - Potentiometric Methods of Analysis
Metallic Indicator Electrodes
The potential of the indicator electrode in a potentiometric electrochemical cell is proportional to the concentration of analyte. Two classes of indicator
electrodes are used in potentiometry: metallic electrodes, which are the sub-
ject of this section, and ion-selective electrodes, which are covered
in the nextsection.
The potential of a metallic electrode is determined by the position of a redox reaction at the electrode–solution
interface. Three types of metallic electrodes
are commonly used in potentiometry, each of which
is considered in the following discussion.
Electrodes of the First Kind
When a copper electrode is immersed in a solution containing Cu2+,
the potential of the electrode due to the
reaction
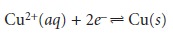
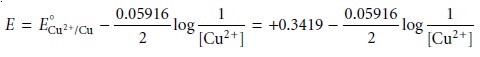
If the copper electrode is the indicator electrode in a potentiometric electrochemical cell that also includes
a saturated calomel
reference electrode
SCE || Cu2+ (unk) | Cu(s)
then the cell potential can be used to determine
an unknown concentration of Cu2+ in the indicator half-cell
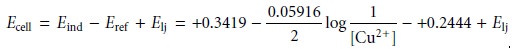
Metallic indicator electrodes in which a metal is in contact
with a solution con- taining its
ion are called
electrodes of the first
kind. In general, for a metal
M, in a solution of Mn+, the cell potential is given as
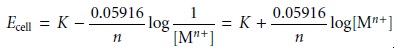
where K is a constant that includes the standard-state potential
for the Mn+/M redox couple,
the potential of the reference electrode, and the
junction potential. For a variety of reasons, including
slow kinetics for electron transfer,
the existence of surface
oxides and interfering reactions, electrodes of the first
kind are limited
to Ag, Bi, Cd,
Cu, Hg, Pb,
Sn, Tl, and
Zn. Many of these electrodes, such as Zn,
cannot be used in acidic solutions where they are
easily oxidized by H+.
Electrodes of the Second Kind
An electrode of the first
kind involving an Mn+/M redox couple will respond to the concentration of another species
if that species is in equilibrium with Mn+. For example,
the potential of a silver
electrode in a solution
of Sg+ is given by
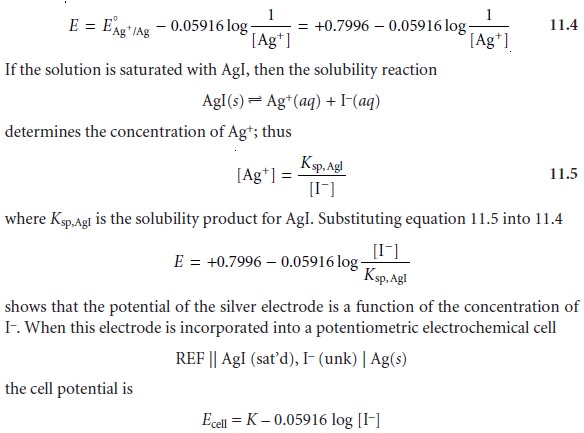
where K
is a constant that includes the standard-state potential for the Ag+/Ag redox
couple, the solubility product for AgI, the potential of the reference
electrode, and the junction potential.
When the potential of an electrode of the first
kind responds to the potential of another ion that is in equilibrium with Mn+, it is called an electrode
of the second kind. Two common
electrodes of the second kind are the calomel and silver/silver
chloride reference electrodes. Electrodes of the second kind also can be based
on complexation reactions. For example, an electrode for EDTA is constructed by cou-
pling a Hg2+/Hg electrode of the first
kind to EDTA
by taking advantage of its for- mation of a stable
complex with Hg2+.
Redox Electrodes
Electrodes of the
first and second
kind develop a potential as the
result of a redox reaction
in which the metallic electrode undergoes a change
in its oxidation state.
Metallic electrodes also can serve
simply as a source of, or a sink for, electrons in other redox
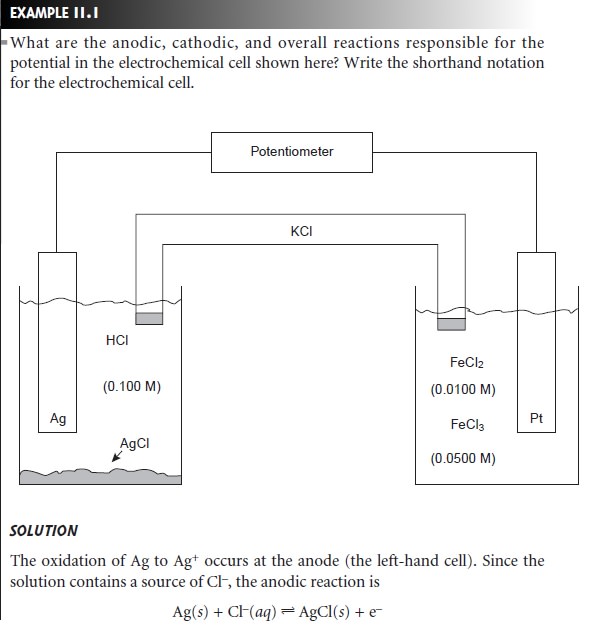
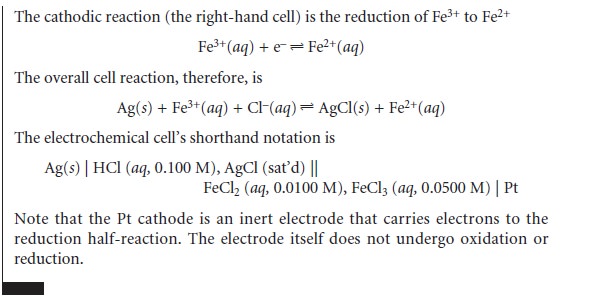
reactions. Such electrodes are called redox electrodes. The Pt cathode in Example 11.1 is an example of a redox electrode because
its potential is determined by the concentrations of Fe2+ and Fe3+ in
the indicator half-cell. Note that the potential of a redox
electrode generally responds to the concentration of more than one ion, limiting
their usefulness for direct potentiometry.
Related Topics