Chapter: Modern Analytical Chemistry: Electrochemical Methods of Analysis
Quantitative and Qualitative Aspects of Voltammetry
Quantitative and Qualitative Aspects of
Voltammetry
Earlier we described
a voltammogram as the electrochemical equivalent of a spec-
trum in spectroscopy. In this section we consider how quantitative and qualitative
information may be extracted from a voltammogram. Quantitative information is
obtained by relating current to the concentration of analyte in the bulk solution.
Qualitative information is obtained from the voltammogram by extracting the standard-state potential for the redox
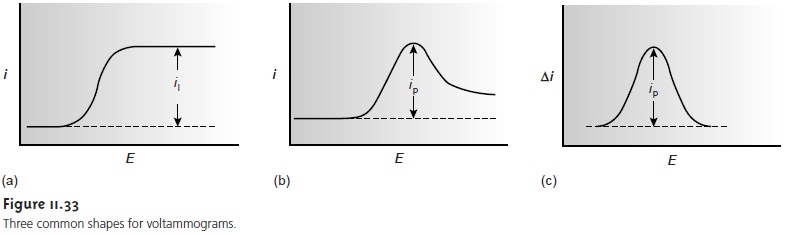
reaction. For simplicity we only consider voltammograms similar
to that shown
in Figure 11.33a.
Determining Concentration
Let’s assume
that the redox
reaction at the working
electrode is
O+
ne– < = = = =
> R ……………11.34
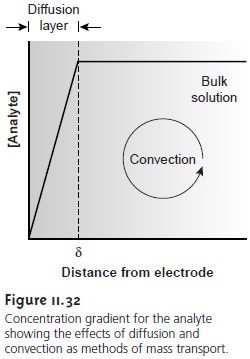
and that initially only O is present in the bulk solution. The current is determined
by the rate at which O diffuses
through the fixed diffusion layer (see Figure 11.32),
and is given by equation
11.33, or
i =
KO([O]bulk – [O]x=0)
……………11.35
where KO is a constant
equal to nFADO/
δ. When the limiting current
is reached, the concentration of O at the electrode surface is zero,
and this equation simplifies to
ilim = KO[O]bulk
……………11.36
Thus, the limiting
current, ilim, is a linear function
of the concentration of O in bulk solution, and a quantitative analysis is possible
using any of the standardization methods. Equations similar
to equation 11.35
can be devel- oped for other forms of voltammetry, in which peak currents are related to the ana- lyte’s concentration in bulk solution.
Determining the Standard-State Potential
To extract the standard-state potential, or formal
potential, for reaction
11.34 from a voltammogram, it is necessary to rewrite the Nernst equation
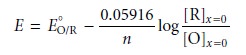 11.37
11.37
in terms of current instead of the concentration of O and R.
Substituting equation 11.36 into equation
11.35 and rearranging gives
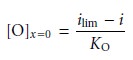 11.38
11.38
To derive a similar equation for the concentration of R at the electrode surface we note that
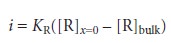
Since the concentration of R in bulk solution is zero, this equation
simplifies to
i = KR[R]x=0
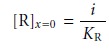 11.38
11.38
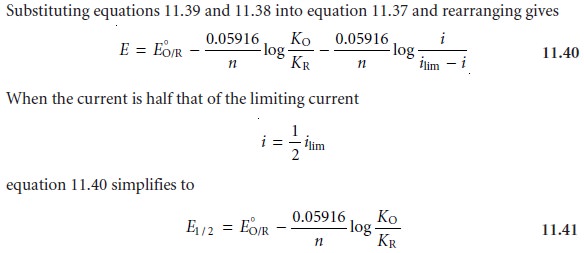
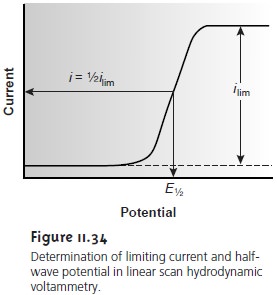
where E1/2 is the half-wave potential (Figure 11.34).
If KO is approximately equal to KR, which is often
the case, then the half-wave potential is equal
to the standard-state potential. Note that equation
11.41 is only valid if the redox reaction is electrochemically
reversible. Voltammetric techniques giv-
ing peak potentials also can
be used to determine a redox reaction’s standard- state potential.
Related Topics