Chapter: Medical Surgical Nursing: Assessment and Management of Patients With Hematologic Disorders
Blood Transfusion Complications
Transfusion Complications
Any
patient who receives a blood transfusion may develop compli-cations from that transfusion.
When explaining the reasons for the transfusion, it is important to include the
risks and benefits and what to expect during and after the transfusion.
Patients must be informed that the supply of blood is not completely risk-free
al-though it has been tested carefully. Nursing management is di-rected toward
preventing complications, promptly recognizing complications if they develop,
and promptly initiating measures to control any complications that occur. The
following sections de-scribe the most common or potentially severe
transfusion-related complications.
FEBRILE, NONHEMOLYTIC REACTION
The nonhemolytic reaction, caused by antibodies to donor WBCs that are still present in the unit of blood or blood compo-nent, is the most common type of transfusion reaction, account-ing for more than 90% of reactions. It occurs more frequently in patients who have had previous transfusions (exposure to multi-ple antigens from previous blood products) and in Rh-negative women who have borne Rh-positive children (exposure to an Rh-positive fetus raises antibody levels in the mother). These reac-tions occur in 1% of PRBC transfusions and 20% of platelet transfusions. More than 10% of patients with a chronic transfu-sion requirement develop this type of reaction.
The
diagnosis of a febrile, nonhemolytic reaction is made by excluding other
potential causes, such as a hemolytic reaction or bacterial contamination of
the blood product. The signs and symptoms of a febrile, nonhemolytic
transfusion reaction are chills (absent to severe) followed by fever (more than
1°C eleva-tion). The fever typically begins within 2
hours after the transfu-sion is begun. Although not life-threatening, the fever
and particularly the chills and muscle stiffness can be frightening to the
patient.
These
reactions can be diminished, even prevented, by further depleting the blood
component of donor WBCs; this is accom-plished by a leukocyte reduction filter.
The blood product may be filtered during processing, which achieves better
results but is more expensive, or during the actual transfusion by adding the
filter to the blood administration tubing. Antipyretics can be given to prevent
fever, but routine premedication is not advised because it can mask the
beginning of a more serious transfusion reaction.
ACUTE HEMOLYTIC REACTION
The
most dangerous, and potentially life threatening, type of trans-fusion reaction
occurs when the donor blood is incompatible with that of the recipient.
Antibodies already present in the recipient’s plasma rapidly combine with
antigens on donor RBCs, and the RBCs are hemolyzed (destroyed) in the
circulation (intravascular hemolysis). The most rapid hemolysis occurs in ABO
incompati-bility. This reaction can occur after transfusion of as little as 10
mL of RBCs. Rh incompatibility often causes a less severe reaction. The most
common causes of acute hemolytic reaction are errors in blood component
labeling and patient identification that result in the administration of an
ABO-incompatible transfusion.
Symptoms
consist of fever, chills, low back pain, nausea, chest tightness, dyspnea, and
anxiety. As the RBCs are destroyed, the hemoglobin is released from the cells
and excreted by the kidneys; therefore, hemoglobin is present in the urine
(hemoglobinuria). Hypotension, bronchospasm, and vascular collapse may result.
Diminished renal perfusion results in acute renal failure, and DIC may also
occur.
The
reaction must be recognized promptly and the transfu-sion discontinued
immediately. Blood and urine specimens must be obtained and analyzed for
evidence of hemolysis. Treatment goals include maintaining blood volume and
renal perfusion and preventing and managing DIC.
Acute
hemolytic transfusion reactions are preventable. Meticulous attention to detail
in labeling blood samples and blood components and identifying the recipient
cannot be overemphasized.
ALLERGIC REACTION
Some
patients may develop urticaria (hives) or generalized itching during a
transfusion. The cause of these reactions is thought to be a sensitivity
reaction to a plasma protein within the blood com-ponent being transfused.
Symptoms of an allergic reaction are ur-ticaria, itching, and flushing. The
reactions are usually mild and respond to antihistamines. If the symptoms
resolve after adminis-tration of an antihistamine (eg, diphenhydramine [eg,
Benadryl]), the transfusion may be resumed. Rarely, the allergic reaction is
se-vere, with bronchospasm, laryngeal edema, and shock. These re-actions are
managed with epinephrine, corticosteroids, and pressor support, if necessary.
Giving
the patient antihistamines before the transfusion may prevent future reactions.
For severe reactions, future blood com-ponents are washed to remove any
remaining plasma proteins. Leukocyte filters are not useful, because the
offending plasma proteins can pass through the filter.
CIRCULATORY OVERLOAD
If too
much blood infuses too quickly, hypervolemia can occur. This condition can be
aggravated in patients who already have in-creased circulatory volume (eg,
those with heart failure). PRBCs are safer to use than whole blood. If the
administration rate is suf-ficiently slow, circulatory overload may be
prevented. For pa-tients who are at risk for, or already in, circulatory
overload, diuretics are administered after the transfusion or between units of
PRBCs. Patients receiving fresh frozen plasma or even platelets may also
develop circulatory overload. The infusion rate of these blood components must
also be titrated to the patient’s tolerance.
Signs
of circulatory overload include dyspnea, orthopnea, tachycardia, and sudden
anxiety. Neck vein distention, crackles at the base of the lungs, and a rise in
blood pressure can also occur. If the transfusion is continued, pulmonary edema
can de-velop, as manifested by severe dyspnea and coughing of pink, frothy
sputum.
If
fluid overload is mild, the transfusion can often be continued after slowing
the rate of infusion and administering diuretics. How-ever, if the overload is
severe, the patient is placed in an upright po-sition with the feet in a
dependent position, the transfusion is discontinued, and the physician is
notified. The intravenous line is kept patent with a very slow infusion of
normal saline solution or a saline or heparin lock device to maintain access to
the vein in case intravenous medications are necessary. Oxygen and morphine may
be needed for severe dyspnea.
BACTERIAL CONTAMINATION
The
incidence of bacterial contamination of blood components is very low; however,
administration of contaminated products puts the patient at great risk.
Contamination can occur at any point dur-ing procurement or processing. Many
bacteria cannot survive in the cold temperatures used to store PRBCs (platelets
are at greater risk for contamination because they are stored at room
tempera-ture), but some organisms can survive cold temperatures.
Preventive
measures include meticulous care in the procure-ment and processing of blood
components. When PRBCs or whole blood is transfused, it should be administered
within a 4-hour pe-riod, because warm room temperatures promote bacterial
growth. A contaminated unit of blood product may appear normal, or it may have
an abnormal color.
The
signs of bacterial contamination are fever, chills, and hypotension. These
signs may not occur until the transfusion is complete, occasionally not until
several hours after the transfu-sion. If the condition is not treated
immediately with fluids and broad-spectrum antibiotics, shock can occur. Even
with aggres-sive management, including vasopressor support, the mortality rate
is high.
As
soon as the reaction is recognized, any remaining trans-fusion is discontinued
and the intravenous line is kept open with normal saline solution. The
physician and the blood bank are notified, and the blood container is returned
to the blood bank for testing and culture. Septicemia is treated with
intra-venous fluids and antibiotics; corticosteroids and vasopressors also may
be necessary.
TRANSFUSION-RELATED ACUTE LUNG INJURY
This
is a potentially fatal, idiosyncratic reaction that occurs in fewer than 1 in
5000 transfusions. Plasma antibodies (usually in the donor’s plasma) that are
present in the blood component stim-ulate the recipient’s WBCs; aggregates of
these WBCs form and occlude the microvasculature within the lungs. This lung
injury is manifested as pulmonary edema; it can occur within 4 hours after the
transfusion.
Signs
and symptoms include fever, chills, acute respiratory dis-tress (in the absence
of other signs of left ventricular failure, such as elevated central venous
pressure), and bilateral pulmonary infiltrates. Aggressive supportive therapy
(oxygen, intubation, diuretics) may prevent death.
DELAYED HEMOLYTIC REACTION
Delayed
hemolytic reactions usually occur within 14 days after transfusion, when the
level of antibody has been increased to the extent that a reaction can occur.
The hemolysis of the RBCs is ex-travascular, via the RES, and occurs gradually.
Signs
and symptoms of a delayed hemolytic reaction are fever, anemia, increased
bilirubin level, decreased or absent haptoglobin, and possibly jaundice. Rarely
is there hemoglobinuria. Generally, these reactions are not dangerous, but it
is useful to recognize them, because subsequent transfusions with blood
products con-taining these antibodies may cause a more severe hemolytic
reac-tion. However, recognition is also difficult, because the patient may not
be in a health care setting to be tested for this reaction, and even if the
patient is hospitalized, the reaction may be too mild to be recognized
clinically. Because the amount of antibody present can be too low to detect, it
is difficult to prevent delayed hemolytic reactions. The reaction is usually
mild and requires no intervention.
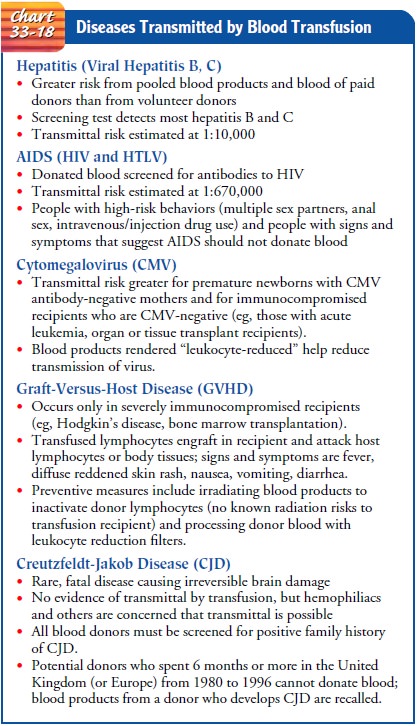
DISEASES TRANSMITTED BY BLOOD TRANSFUSION
Despite
the advances in donor screening and blood testing, cer-tain diseases can still
be transmitted by transfusion of blood components. The diseases in Chart 33-18
are examples of this phenomenon.
COMPLICATIONS OF LONG-TERM TRANSFUSION THERAPY
The
complications that have been described represent a real risk for any patient
any time a unit of blood is administered. How-ever, patients with long-term
transfusion therapy (eg, those with MDS, thalassemia, sickle cell anemia) are
at greater risk for in-fection transmission and for becoming more sensitized to
donor antigens, simply because they are exposed to more units of blood and,
consequently, more donors. Iron overload is a complication unique to those
individuals with long-term PRBC transfusions. A summary of complications
associated with long-term transfusion therapy is depicted in Table 33-11.
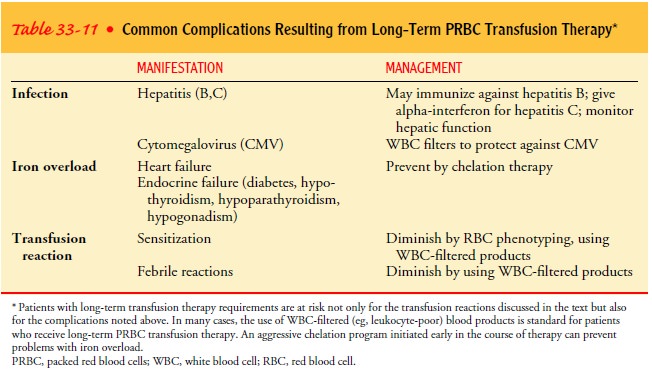
Iron Overload. One unit of PRBCs contains 250 mg of iron.Patients with chronic transfusion requirements can quickly acquire more iron than they can use, leading to iron overload. Over time, the excess iron deposits in the tissues and can cause organ damage, particularly in the liver, heart, testes, and pancreas. Promptly initiating a program of iron chelation therapy (eg, with deferoxamine [Desferal]) can prevent end-organ damage from iron toxicity (Giardina & Grady, 1995).
NURSING MANAGEMENT FOR TRANSFUSION REACTIONS
If a
transfusion reaction is suspected, the transfusion must be im-mediately stopped
and the physician notified. A thorough patient assessment is crucial, because
many complications have similar signs and symptoms. The following steps are
taken to determine the type and severity of the reaction:
•
Stop the transfusion. Maintain the intravenous line
with normal saline solution through new intravenous tubing, administered at a
slow rate.
•
Assess the patient carefully. Compare the vital
signs with those from the baseline assessment. Assess the patient’s
res-piratory status carefully. Note the presence of adventitious breath sounds,
use of accessory muscles, extent of dyspnea
•
(if any), and changes in mental status, including
anxiety and confusion. Note any chills, diaphoresis, complaints of back pain,
urticaria, and jugular vein distention.
•
Notify the physician of the assessment findings,
and imple-ment any orders obtained. Continue to monitor the patient’s vital
signs and respiratory, cardiovascular, and renal status.
•
Notify the blood bank that a suspected transfusion
reaction has occurred.
•
Send the blood container and tubing to the blood
bank for repeat typing and culture. The identifying tags and numbers are
verified.
If a
hemolytic transfusion reaction or bacterial infection is sus-pected, the nurse
should do the following:
•
Obtain appropriate blood specimens from the
patient.
•
Collect a urine sample as soon as possible for a hemoglobin
determination.
•
Document the reaction, according to the
institution’s policy.
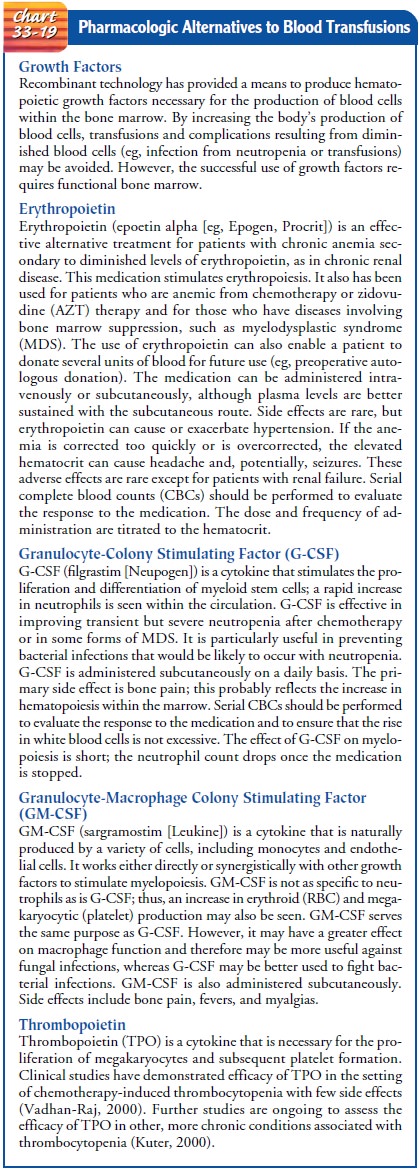
PHARMACOLOGIC ALTERNATIVES TO BLOOD TRANSFUSIONS
Pharmacologic
agents to stimulate production of one or more types of blood cells by the
marrow are commonly used. Chart 33-19 pre-sents examples of such pharmacologic
agents.
Researchers
continue to seek a blood substitute that is prac-tical and safe. Blood
substitutes previously tried have not been successful. However, newer blood
substitutes focus solely on oxygen delivery, as an RBC substitute (Rabinovici,
2001). Cur-rent blood substitutes in clinical trials have distinct advantages
and disadvantages compared with human RBCs. They are man-ufactured hemoglobin
solutions that can be sterilized without destroying the blood substitute. They
require no refrigeration and appear to have a long shelf-life (possibly 1 year,
versus lit-tle more than 1 month for PRBCs). Perhaps more importantly, they
require no cross-matching, because there is no RBC mem-brane to interact with antibodies
in the recipient’s serum. The most significant disadvantage stems from the
blood substitutes extremely short life within human circulation—approximately 1
day, instead of the 30-day life span of a conventionally transfused RBC.
Therefore, the use of these products would likely be limited to situations in
which the need is short-term (eg, surgery, trauma). Finally, the blood
substitutes are likely to be extremely expensive.
PERIPHERAL BLOOD STEM CELL TRANSPLANTATION (PBSCT) AND BONE MARROW TRANSPLANTATION (BMT)
PBSCT
and BMT are therapeutic modalities that offer the pos-sibility of cure for some
patients with hematologic disorders such as severe aplastic anemia, some forms
of leukemia, and thal-assemia. Because most hematologic disease states arise from
some form of bone marrow dysfunction, an autologous trans-plantation (receiving
one’s own stem cells) is not as common an option as is allogeneic
transplantation. A patient receives inten-sive chemotherapy (sometimes with
radiation therapy as well), with the goal being complete ablation of the
patient’s bone mar-row. Stem cells from the donor (ideally, from a matched
sibling), or actual marrow from the donor, is then infused into the patient
using a process similar to an RBC transfusion. The stem cells travel to the
marrow and slowly begin the process of resuming hematopoiesis. The advantage of
autotransplantation is the re-duced likelihood of complications and mortality;
however, the risk of relapse is also higher.
A
relatively new strategy is based on transplantation for adop-tive cell therapy
using certain immune mechanisms derived from the donor’s lymphocytes (Slavin et
al., 2001; Margolis, Borrello, & Flinn, 2000). In nonmyeloablative stem
cell or marrow transplantation, also referred to as a “minitransplant,” the
conditioning regimen involves much less myelosuppression than in conventional
regimens, rendering the patient immuno-suppressed but for a shorter period of
time. Consequently, the procedure is less toxic to the patient, and there is a
significant decrease in morbidity.
After
the deconditioning regimen (ie, during the time the pa-tient is
immunosuppressed), the allotransplantation is performed, using either marrow or
stem cells. The goal is for the donor’s lym-phocytes to react against any residual
malignant cells within the patient and destroy them. This process is typically
augmented byinfusion of the donor’s lymphocytes as well (referred to as donor
lymphocyte infusion, or DLI). If relapse occurs, repeated DLI has been
effective in reestablishing remission in many patients. This approach has great
promise, particularly in the setting of hema-tologic malignancy, and may
provide a mechanism to increase the utility of transplantation for more
patients than is possible with conventional methods.
Success of transplantation depends on tissue compatibility and the patient’s tolerance of the immunosuppression that re-sults from the ablative therapy. Patients require intensive nurs-ing care that is directed toward preventing infection and assessing for early signs and symptoms of complications. One common complication involves the formation of lymphocytes that respond to their new host (ie, the patient) as foreign and mount a reaction against the body. This process, known as graft-versus-host disease (GVHD), can involve the skin, gastrointesti-nal tract, and liver and can be life-threatening. In hematologic malignancies, some GVHD is actually desirable in that the donor lymphocytes can also mount a reaction against any lin-gering tumor cells; this process is referred to as graft-versus-malignancy. GVHD is a significant complication in nonmye-loablative transplantation therapy, as well as in conventional al-lotransplantation. Late complications (occurring more than 100 days after transplantation) are frequent; these patients, particu-larly those who receive an allogeneic transplant, require careful follow-up for years after transplantation.
Related Topics