Chapter: Medical Surgical Nursing: Assessment and Management of Patients With Hematologic Disorders
Blood Cells - Anatomic and Physiologic Overview
BLOOD
CELLS
Red Blood Cells (RBCs)
The
normal RBC is a biconcave disk that resembles a soft ball compressed between
two fingers (Fig. 33-2). It has a diameter of about 8 ÎĽ m and
is so flexible that it can pass easily through capil-laries that may be as
small as 2.8 ÎĽ m in diameter. The RBC mem-brane is so thin
that gases, such as oxygen and carbon dioxide, can easily diffuse across it;
the disk shape provides a large surface area that facilitates the absorption
and release of oxygen molecules.
Mature
RBCs consist primarily of hemoglobin,
which contains iron and makes up 95% of the cell mass. RBCs have no nuclei, and
they have many fewer metabolic enzymes than do most other cells. The presence
of a large amount of hemoglobin enables the RBC to perform its principal
function, the transport of oxygen between the lungs and tissues. Occasionally
the marrow releases slightly imma-ture forms of RBCs, called reticulocytes, into the circulation.
This occurs as a normal response to an increased demand for RBCs (as in
bleeding) or in some disease states.
The
oxygen-carrying hemoglobin molecule is made up of four subunits, each
containing a heme portion attached to a globin chain. Iron is present in the
heme component of the molecule. An important property of heme is its ability to
bind to oxygen loosely and reversibly. Oxygen readily binds to hemoglobin in
the lungs and is carried as oxyhemoglobin
in arterial blood. Oxyhemoglo-bin is a brighter red than hemoglobin that does
not contain oxy-gen (reduced hemoglobin), which is why arterial blood is a
brighter red than venous blood. The oxygen readily dissociates (detaches) from
hemoglobin in the tissues, where the oxygen is needed for cellular metabolism.
In venous blood, hemoglobin combines with hydrogen ions produced by cellular
metabolism and thus buffers excessive acid. Whole blood normally contains about
15 g of hemoglobin per 100 mL of blood.
ERYTHROPOIESIS
Erythroblasts
arise from the primitive myeloid stem cells in bone marrow. The erythroblast is
a nucleated cell that, in the process of maturing within the bone marrow,
accumulates hemoglobin and gradually loses its nucleus. At this stage, the cell
is known as a retic-ulocyte. Further maturation into an RBC entails the loss of
the dark-staining material and slight shrinkage. The mature RBC is then
released into the circulation. Under conditions of rapid erythropoiesis (RBC production), reticulocytes and other imma-ture
cells (eg, nucleated RBCs) may be
released prematurely into the circulation.
Differentiation
of the primitive myeloid stem cell of the mar-row into an erythroblast is
stimulated by erythropoietin, a
hor-mone produced primarily by the kidney. If the kidney detects low levels of
oxygen (as would occur in anemia, in
which fewer RBCs are available to bind oxygen, or in people living at high
altitudes), the release of erythropoietin is increased. The increased
erythro-poietin then stimulates the marrow to increase production of RBCs. The
entire process typically takes 5 days.
For
normal RBC production, the bone marrow also requires iron, vitamin B12, folic acid, pyridoxine
(vitamin B6), protein, and other
factors. A deficiency of these factors during erythropoiesis can result in
decreased RBC production and anemia.
Iron Stores and Metabolism.
The average
daily diet in the UnitedStates contains 10 to 15 mg of elemental iron; normally
0.5 to 1 mg of ingested iron is absorbed from the small intestine. The rate of
iron absorption is regulated by the amount of iron already stored in the body
and by the rate of RBC production. Additional amounts of iron, up to 2 mg
daily, must be absorbed by women to replace blood lost during menstruation.
Total body iron content in the average adult is approximately 3 g, most of
which is present in hemoglobin
or in one of its breakdown products. Iron is stored in the small intestine as
ferritin and in reticuloendothelial cells. When required, the iron is released
into the plasma, binds to transferrin, and is transported into the membranes of
the normoblasts (RBC precursor cells) within the marrow, where it is
incorporated into hemoglobin. Iron is lost in the feces, either in bile, blood,
or mu-cosal cells from the intestine.
The
concentration of iron in blood is normally about 75 to 175 ÎĽ g/dL (13 to 31 ÎĽ
mol/L) for men and 65 to 165 ÎĽ g/dL (11 to 29 ÎĽ mol/L) for women. With iron deficiency, bone
marrow iron stores are rapidly depleted; hemoglobin synthesis is depressed, and
the RBCs produced by the marrow are small and low in he-moglobin. Iron
deficiency in the adult generally indicates that blood has been lost from the
body (eg, from bleeding in the gas-trointestinal tract or heavy menstrual
flow). In the adult, lack of dietary iron is rarely the sole cause of iron
deficiency anemia. The source of iron deficiency should be investigated
promptly, because iron deficiency in an adult may be a sign of bleeding in the
gastro-intestinal tract or colon cancer.
Vitamin B12 and Folic Acid Metabolism.
Vitamin B12and folicacid are required for
the synthesis of DNA in many tissues, but de-ficiencies of either of these
vitamins have the greatest effect on erythropoiesis. Both vitamin B12 and folic
acid are derived from the diet. Folic acid is absorbed in the proximal small
intestine, but only small amounts are stored within the body. If the diet is
defi-cient in folic acid, stores within the body quickly become depleted.
Because vitamin B12 is found only in foods of animal
origin, strict vegetarians may ingest little B12. Vitamin B12 combines
with in-trinsic factor produced in the stomach. The vitamin B–intrinsic factor
complex is absorbed in the distal ileum. People who have had a partial or total
gastrectomy may have limited amounts of in-trinsic factor, and therefore the
absorption of B12 may be dimin-ished. The effects of either decreased absorption or
decreased intake of B12 are not apparent for 2 to 4
years.
Vitamin
B12 and folic acid
deficiencies are characterized by the production of abnormally large RBCs
called megaloblasts. Because these cells are abnormal, many are sequestered
(trapped) while still in the bone marrow, and their rate of release is
de-creased. Some of these cells actually die in the marrow before they can be
released into the circulation. This results in megaloblastic anemia.
RED BLOOD CELL DESTRUCTION
The
average life span of a normal circulating RBC is 120 days. Aged RBCs lose their
elasticity and become trapped in small blood vessels, particularly in the
spleen. They are removed from the blood by the reticuloendothelial cells,
particularly in the liver and the spleen. As the RBCs are destroyed, their
hemoglobin is largely recycled. Some hemoglobin also breaks down to form
bilirubin and is secreted in the bile. Most of the iron is recycled to form new
hemoglobin molecules within the bone marrow; small amounts are lost daily in
the feces and urine and monthly in menstrual flow.
White Blood Cells (WBCs)
Leukocytes
are divided into two general categories: granulocytes and lymphocytes. In
normal blood, the total leukocyte count is 5000 to 10,000 cells per cubic
millimeter. Of these, approxi-mately 60% to 70% are granulocytes and 30% to 40%
are lym-phocytes. Primarily, WBCs protect the body against infection and tissue
injury.
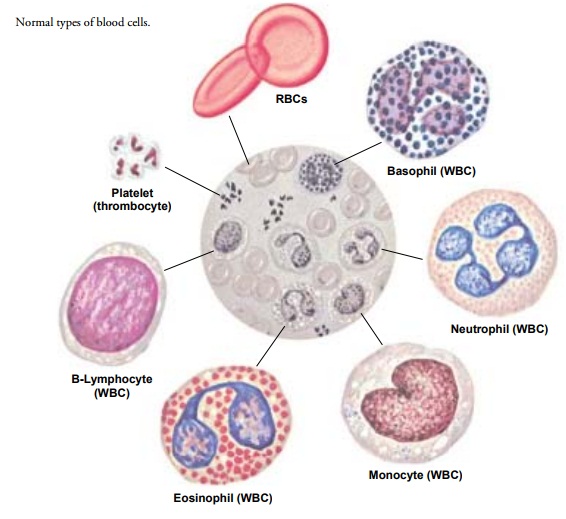
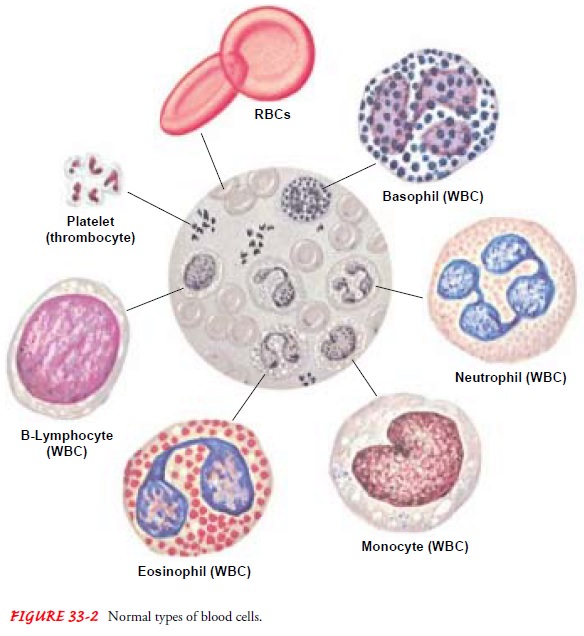
GRANULOCYTES
Granulocytes are defined by the presence of granules in
the cyto-plasm of the cell. Granulocytes are divided into three main
sub-groups, which are characterized by the staining properties of these
granules (see Fig. 33-2). Eosinophils have bright-red granules in their
cytoplasm, whereas the granules in basophils stain deep blue. The third and by
far the most numerous cell in this class is the neutrophil, with granules that
stain a pink to violet hue. Neu-trophils are also called polymorphonuclear
neutrophils (PMNs, or polys) or segmented neutrophils (segs).
The
nucleus of the mature neutrophil has multiple lobes (usu-ally two to five) that
are connected by thin filaments of nuclear ma-terial, a “segmented” nucleus; it
is usually twice the size of an RBC. The somewhat less mature granulocyte has a
single-lobed, elon-gated nucleus and is called a band cell. Ordinarily, band cells ac-count for only a small
percentage of circulating granulocytes, although their percentage can increase
greatly under conditions in which neutrophil production increases, such as
infection. An in-creased number of band cells is sometimes called a “left shift” or “shift to the left.” (Traditionally, the diagram of neutrophil
mat-uration shows the stem cell on the left with progressive maturation stages
toward the right, ending with a fully mature neutrophil on the right side. A
shift to the left indicates that more immature cells are present in the blood
than normally occurs.)
Granulocyte production from the myeloid stem
cell pool results in the gradual differentiation of these cells from a myeloid blast cell into a fully mature
neutrophil. The process, called myelopoiesis,
is highly complex and depends on many factors. These factors, in-cluding
specific cytokines such as growth
factors, are normally present within the marrow itself. As the blast cell
matures, the cy-toplasm of the cell changes in color (from blue to violet) and
gran-ules begin to form with the cytoplasm. The shape of the nucleus also
changes. The entire process of maturation and differentiation takes about 10 days
(see Fig. 33-1). Once the neutrophil is released into the circulation from the
marrow, it stays there for only about 6 hours before it migrates into the body
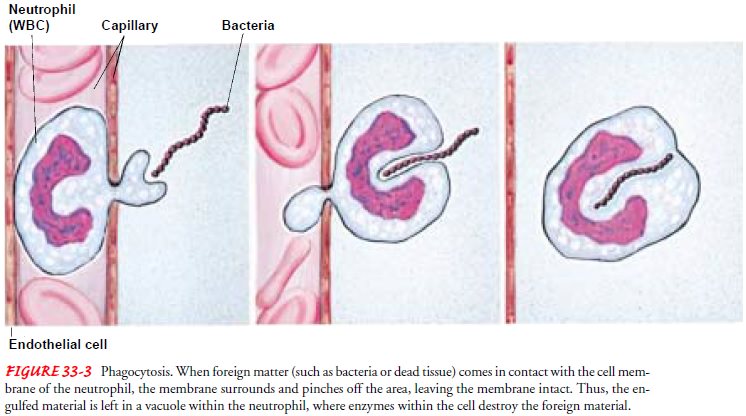
tissues to perform its func-tion of phagocytosis
(ingestion and digestion of bacteria and par-ticles) (Fig. 33-3). Here,
neutrophils last no more than 1 to 2 days before they die. The number of
circulating granulocytes found in the healthy person is relatively constant,
but in infection large num-bers of these cells are rapidly released into the circulation.
MONONUCLEAR WHITE BLOOD CELLS (AGRANULOCYTES)
Monocytes.
Monocytes(also called mononuclear
leukocytes) areWBCs with a single-lobed nucleus and a granule-free cytoplasm—
hence the term agranulocyte. In
normal adult blood, monocytes ac-count for approximately 5% of the total WBCs.
Monocytes are the largest of the WBCs. Produced by the bone marrow, they remain
in the circulation for a short time before entering the tissues and
transforming into macrophages.
Macrophages are particularly active in the spleen, liver, peritoneum, and the
alveoli of the lungs.
Lymphocytes.
Mature lymphocytes are small cells with scantycytoplasm. Immature lymphocytes are produced in the marrow from the lymphoid stem cells. A second major source of produc-tion is the cortex of the thymus. Cells derived from the thymus are known as T lymphocytes (or T cells); those derived from the marrow can also be T cells but are more commonly B lympho-cytes (or B cells). Lymphocytes complete their differentiation and maturation primarily in the lymph nodes and in the lymphoid tissue of the intestine and spleen after exposure to a specific anti-gen. Mature lymphocytes are antigen-specific cells.
FUNCTION OF WHITE BLOOD CELLS
WBCs
protect the body from invasion by bacteria and other for-eign entities. The
major function of neutrophils is phagocytosis (see Fig. 33-3). Neutrophils
arrive at the site within 1 hour after the onset of an inflammatory reaction
and initiate phagocytosis, but they are short-lived. An influx of monocytes
follows; these cells continue their phagocytic activities for long periods as
macrophages. This process constitutes a second line of defense for the body
against inflammation and infection. Although neu-trophils can often work
adequately against bacteria without the need for excessive involvement with
macrophages, macrophages are particularly effective against fungi and viruses.
Macrophages also digest senescent (aging or aged) blood cells, such as RBCs,
primarily within the spleen.
The
primary function of lymphocytes is to produce substances that aid in attacking
foreign material. One group of lymphocytes (T lymphocytes) kills foreign cells
directly or releases a variety of lymphokines, substances that enhance the
activity of phagocytic cells. T lymphocytes are responsible for delayed
allergic reactions, rejection of foreign tissue (eg, transplanted organs), and
destruc-tion of tumor cells. This process is known as cellular immunity. The other group of lymphocytes (B lymphocytes)
is capable of differ-entiating into plasma cells. Plasma cells, in turn,
produce im-munoglobulin (Ig), or antibodies, which are protein molecules that
destroy foreign material by several mechanisms. This process is known as humoral immunity.
Eosinophils
and basophils function in hypersensitivity reactions. Eosinophils are important
in the phagocytosis of parasites. The in-crease in eosinophil levels in
allergic states indicates that these cells are involved in the hypersensitivity
reaction; their function there is to neutralize histamine. Basophils produce
and store histamine as well as other substances involved in hypersensitivity
reactions. The release of these substances provokes allergic reactions.
Platelets (Thrombocytes)
Platelets,
or thrombocytes, are not actually cells. Rather, they are granular fragments of
giant cells in the bone marrow called megakaryocytes. Platelet production in
the marrow is regulated in part by the hormone thrombopoietin, which stimulates
the production and differentiation of megakaryocytes from the myeloid stem
cell.
Platelets
play an essential role in the control of bleeding. They circulate freely in the
blood in an inactive state, where they nur-ture the endothelium of the blood
vessels, maintaining the in-tegrity of the vessel. When vascular injury does
occur, platelets collect at the site and are activated. They adhere to the site
of in-jury and to each other, forming a platelet plug that temporarily stops
bleeding. Substances released from platelet granules activate coagulation
factors in the blood plasma and initiate the formation of a stable clot
composed of fibrin, a filamentous
protein. Platelets have a normal life span of 7 to 10 days.
Related Topics