Physics - Atomic and Nuclear Physics | 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Chapter: 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Atomic and Nuclear Physics
ATOMIC AND NUCLEAR PHYSICS
LEARNING OBJECTIVES
In this unit, the students are
exposed to
• electric discharge through the gases
• determination of specific charge by J.J. Thomson
experiment
• determination of electronic charge by Millikan’s
oil drop experiment
• atom models – J.J. Thomson and Rutherford
• Bohr atom model and hydrogen atom
• atomic spectrum and hydrogen spectrum
• structure and properties of nucleus
• various classification of nuclei based on atomic
and mass number
• mass defect and binding energy
• relation between stability and binding energy
curve
• alpha, beta and gamma decay
• law of radioactive decay
• nuclear fission and fusion
• elementary ideas of nuclear reactors
• qualitative idea of elementary particles
INTRODUCTION
In earlier classes, we have studied that anything which occupies space is called matter. Matter can be classified into solids, liquids and gases. In our daily life, we use water for drinking, petrol for vehicles, we inhale oxygen, stainless steel vessels for cooking, etc. Experiences tell us that behaviour of one material is not same as another, this means that the physical and chemical properties are different for different materials. In order to understand this, we need to know the fundamental constituents of materials.
When an object is divided
repeatedly, the process of division could not be done beyond a certain stage in
a similar way and we end up with a small speck. This small speck was defined as
an atom. The word atom in Greek means ‘without division or indivisible’. The
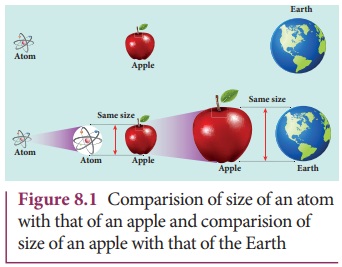
size of an atom is very very small. For an example, the size of hydrogen atom
(simplest among other atoms) is arround 10–10 m. An American Physicist Richard
P. Feynman said that if the atom becomes the size of an apple, then the apple
becomes the size of the earth as shown in Figure 8.1. Such a small entity is an
atom.
In this unit, we first discuss the
theoretical models of atom to understand its structure. The Bohr atom model is
more successful than J. J. Thomson and Rutherford atom models. It explained
many unsolved issues in those days and also gave better understanding of
chemistry.
Later, scientists observed thateven
theatom is not the fundamental entity. It consists of electrons and nucleus.
Around 1930, scientists discovered that nucleus is also made of proton and
neutron. Further research discovered that even the proton and neutron are made
up of fundamental entities known as quarks.
In this context, the remaining part
of this unit is written to understand the structure and basic properties of
nucleus. Further how the nuclear energy is produced and utilized are discussed.
Related Topics