Atomic and Nuclear Physics | Physics - Short answer questions | 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Chapter: 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Short answer questions
II Short answer questions
1. What are cathode rays?.
Cathode rays are streams of electrons emitting from cathode in a Coolidge tube at a
pressure of around 0.01 mm of Hg.
2. Write the properties of cathode rays.
1)
Cathode rays possess energy and momentum
2)
They travel in a straight line
3)
They are deflected by electric and
magnetic fields
4)
They affect photographic plates
5)
They produce fluorescence when they
fall on certain crystals and minerals
6)
They ionize the gas through which
they pass
7)
They produce heat, when they fall on
matter
8)
They are negatively charged
particles nothing but electrons
9)
When the cathode rays fall on a material of high atomic weight, X-rays are produced.
10)
The speed of cathode rays is upto (1/10)th of the
speed of light.
3. Give the results of Rutherford alpha scattering experiment.
1)
Most of the alpha particles are undeflected
through the gold foil and went straight
2)
Some alpha particles are deflected
through a small angle
3) A few alpha
particles are deflected through the angle more
that 900.
4)
Very few alpha particles returned
back ie., deflected back by 180°.
4. Write down the postulates of Bohr atom model.
1)
The electron in an atom moves around nucleus in circular orbits under the influence of Coulomb electrostatic force of attraction. This force gives
necessary centripetal force for the electron to undergo
circular motion.
2)
Electrons in an atom revolve around the nucleus only in certain discrete orbits
which does not readiate electromagnetic energy called stationary orbits. The
angular momentum of the electrons in these stable orbits are quantized ie.,
angular momentum equal to integral multiple of h/2π
l =
nh / 2π
This is
Angular momentum Quantization condition
3)
Energy of orbits are not continuous but only discrete ie, Quantization of
energy.
4)
An electron can jump from one orbit to another orbit by absorbing or emitting a
photon whose energy is equal to the difference in energy (∆E) between the two
orbital levels.
ΔE
= Efinal – Einitial = hυ = hc / λ
υ – Frequency; h-Planck’s constant
5. What is meant by excitation energy.
The
energy required to excite an electron from lower energy state to any higher
energy state is known as excitation energy.
6. Define the ionization energy and ionization potential.
❖ The minimum energy required to
remove an electron from an atom in the ground state is known as binding energy
or ionization energy.
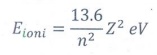
❖ Ionization potential is defined
as ionization energy per unit charge
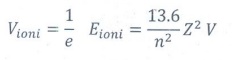
7. Write down the draw backs of Bohr atom model.
The
following are the drawbacks of Bohr atom model
1)
Bohr atom model is valid only for hydrogen
atom or hydrogen like-atoms but not for complex atoms.
2)
When the spectral lines are closely examined, individual lines of hydrogen
spectrum is accompanied by a number of faint lines. These are often called fine structure. This can not explained by Bohr atom model.
3)
Bohr atom model fails to explain the intensity
variations in the spectral lines.
4)
The distribution of electrons in
atoms is not completely explained by Bohr atom model.
8. What is distance of closest approach?
The
minimum distance or contact distance between the centre of the nucleus and the
alpha particle just before it gets reflected back through 180° is defined as
the distance of closest approach (contact distance).
r0 = 1/4πεo
[ 2ze2/Ek ]
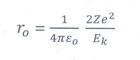
9. Define impact parameter.
The
impact parameter is defined as the perpendicular distance between the centre of
the gold nucleus and the direction of velocity vector of alpha particle when it
is at a large distance.
b
= Kcot (θ/2 )
where
K = 1/ 4πε 0 [ 2Ze2
/ mv20
]
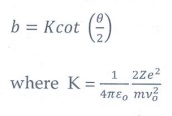
10. Write a general notation of nucleus of element X. What each term denotes?
General
notation of nucleus of element AZX
X
- Chemical symbol of the element
A
- Mass number
Z
- Atomic Number
N
- Number of neutrons (N = A-Z)
11. What is isotope? Give an example.
Isotopes are atoms of the same element having same atomic number but different mass number.
Ex
: 11H - Hydrogen, - 21H - Deuterium,
31H -
Tritium and 116C, 126C, 136C,
146C
12. What is isotone? Give an example.
Isotones
are the atoms of the different elements having same number of neutrons.
Ex
: 125B – Boron, 136C – Carbon. Both
have 7 neutrons
13. What is isobar? Give an example.
Isobars are the
atoms of different elements having the same mass number but different atomic
number.
Ex:
4018Ar – Argon, 4020Ca - Calcium
14. Define atomic mass unit u.
One
atomic mass unit is defined as the 1/12th
of the mass of the isotope of
carbon 126C.
1
u = 1.66 x 10-27 kg
15. Show that nuclear density is almost constant for nuclei with Z > 10.
Nuclear
density is defined as the ratio of the mass of the nuclei to the volume of the
nuclei.
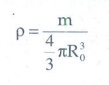
It
shows that density is independent of mass number. In other words all the nuclei
(z >10) have the same density.
ρ = 2.3 x 1017
kg m-3
16. What is mass defect?
The
difference in the total mass of the nucleons and the actual mass of the nucleus
is known as the mass defect.
Δm = (Zmp + Nmn) - M
M
- Actual mass of the nucleus
17. What is binding energy of a nucleus? Give its expression.
When
the protons and neutrons combine to form a nucleus, the mass that disappears is
converted into an equivalent amount of energy. This energy is called the binding energy of the nucleus.
BE
= (Zmp + Nmn - MA)c2
=
Δmc2
18. Calculate the energy equivalent of 1 atomic mass unit.
1u = 1.67 × 10-27 kg
Energy
equivalent of lu = u × c2
=
1.66 × 10-27 x ( 3× l08 )2
=
1.66 × l0-27 × 9 × 1016
=
14.94 × 10-11 J
=
14.94 × 10-11 /1.6 × 10-19
eV
=
931 × 106 eV = 931 MeV
Energy equivalent of l u = 931
MeV
19. Give the physical meaning of binding energy per nucleon.
The
average binding energy per nucleon is the energy required to separate a single
nucleon from the particular nucleus.
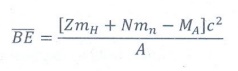
20. What is meant by radioactivity?
The
phenomenon of spontaneous emission of highly penetrating radiations such as α,β
and γ rays by an element is called radioactivity
and the substances which emit these radiations are called radioactive elements.
21. Give the symbolic representation of alpha decay, beta decay and gamma decay.

22. In alpha decay, why the unstable nucleus emits 42He nucleus? Why it does not emit four separate nucleons?
42He
consists of two protons and two neutrons. For example if 23892U
nucleus decays into 23490Th by emitting four separate
nucleons (two protons and two neutrons), then the disintegration energy Q is
negative.
It
implies that the total mass of products is greater than that of parent (23892U)
nucleus.
This
kind of process cannot occur in nature because it would violate conservation of
energy. In any decay process
❖ conservation of energy
❖ conservation of linear momentum
❖ conservation of angular momentum
must be obeyed. So, the unstable nucleus emits 42He
nucleus.
23. What is mean life of radioactive nucleus? Give the expression.
The
mean life time of the nucleus is the ratio of sum of life times of all nuclei
to the total number of nuclei present initially
τ = 1/ λ =1.443 T 1 /2
Mean
life time is also equal to reciprocal of decay constant.
24. What is half-life of nucleus? Give the expression.
The
half life (T1/2) is defined as the time required for the number of
atoms initially present to reduce to one half of the initial amount.
T1/2= 0.6931/ λ
25. What is meant by activity or decay rate? Give its unit.
Activity
is defined as the number of nuclei decayed per second.
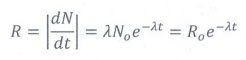
R=λN
SI
unit of activity is Becquerel (Bq) and standard unit is Curie (Ci).
26. Define curie.
One
curie is defined as the number of decays per second in lg of radium and it is
equal to
3.7
x 1010 decays/s.
1 Ci = 3.7 x 1010 Bq
27. What are the constituent particles of neutron and proton?
❖ Neutron consists of one up quark
and two down quarks.
❖ Proton consists of one down
quark and two up quarks.
Related Topics