Radioactivity | Nuclear Physics - Carbon dating | 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Chapter: 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Carbon dating
Carbon dating
The interesting application of beta
decay is radioactive dating or carbon dating. Using this technique, the age of
an ancient object can be calculated. All living organisms absorb carbon dioxide
(CO2) from air to synthesize organic molecules. In this absorbed CO2,
the major part is 126C
and very small fraction (1.3 ×10-12 ) is radioactive 146C whose half-life is 5730 years.
Carbon-14 in the atmosphere is
always decaying but at the same time, cosmic rays from outer space are
continuously bombarding the atoms in the atmosphere which produces 146C . So the continuous production and decay
of 146C in the atmosphere
keep the ratio of 146C
to 126C always constant. Since our human body, tree or
any living organism continuously absorb CO2 from the atmosphere, the
ratio of 146C to
126C in the
living organism is also nearly constant. But when the organism dies, it stops
absorbing CO2. Since 146C starts to decay, the ratio of 146C
to 612C in a dead organism or specimen decreases over the
years. Suppose the ratio of 146C to 126C
in the ancient tree pieces excavated is known, then the age of the tree pieces
can be calculated.
EXAMPLE 8.14
Keezhadi (கீழடி), a small hamlet, has become one of the very important archeological
places of Tamilandu. It is located in Sivagangai district. A lot of tools, jewellery
and charcoal, etc.) have been unearthed in Keezhadi which have given
substantial evidence that an ancient urban civilization had thrived on the
banks of river Vaigai. To determine the age of those
materials, the charcoal of 200 g sent for carbon dating is given in the
following figure (b). The activity of 146C is found to be 38 decays/s. Calculate
the age of charcoal.

Solution
To calculate the age, we need to
know the initial activity (R0) of the characol (when the sample was
alive).
The activity R of
the sample
R = R0 e−λt (1)
To find the time t, rewriting the above equation (1), eλt = R0/R
By taking the logarithm on both sides, we get

Here R = 38 decays/s=38 Bq.
To find decay constant, we use the equation
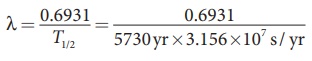
[∴ 1yr =
365.25 × 24 × 60 × 60 s = 3.156 × 107 s]
λ = 3.83×10−12
s−1
To find the initial activity R0, we use the equation R0 = λN0 . Here N0
is the number of carbon-14 atoms present in the sample when it was alive. The
mass of the characol is 200 g. In 12 g of carbon, there are 6.02 ×1023 carbon
atoms. So 200 g contains,
[{ 6.02×1023 atoms / mol } / {12g / mol } ] × 200 ≈ 1×1025 Atoms
When the tree(sample) was alive, the
ratio of 146C to
126C is 1.3 ×10-12
. So the total number of carbon-14 atoms is given by
N0 = 1×1025×1.3×10−12
= 1.3×1013 atoms
The initial activity
R0 = 3.83×10−12
×1.3×1013 ≈ 50 decays / s
= 50 Bq
By substituting the value of R0 and λ in the equation (2),
we get
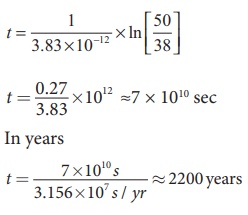
In fact, the excavated materials
were sent for carbon dating to USA by Archeological Department of Tamilnadu and
the report confirmed that the age of Keezhadi artefacts lies between 2200 years
to 2500 years (Sangam era- 400 BC to 200 BC). The Keezhadi excavations
experimentally proved that urban civilization existed in Tamil Nadu even 2000
years ago!
Related Topics