Nuclear Physics - Atomic and nuclear masses | 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Chapter: 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Atomic and nuclear masses
Atomic and
nuclear masses
The mass of nuclei is very small when it expressed in SI units
(about 10-25 kg or less). Therefore, it is more convenient to
express it in terms of another unit namely, the atomic mass unit (u). One
atomic mass unit (u) is defined as
the 1/12th of the mass of the isotope of carbon 126C ,
the most abundant naturally occurring isotope of carbon.
In other words
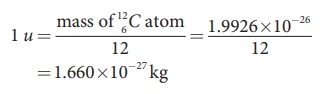
In terms of this atomic mass unit, the mass of the neutron =
1.008665 u, the mass of the proton
=1.007276 u, the mass of the hydrogen
atom = 1.007825 u and the mass of 126C = 12u. Note that usually mass specified is the mass of the atoms, not
mass of the nucleus. To get the nuclear mass of particular nucleus, the mass of
electrons has to be subtracted from the corresponding atomic mass.
Experimentally the atomic mass is determined by the instrument called
Bainbridge mass spectrometer. If we determine the atomic mass of the element
without considering the effect of its isotopes, we get the mass averaged over
different isotopes weighted by their abundances.
EXAMPLE 8.6
Calculate the average atomic mass of
chlorine if no distinction is made between its different isotopes?
Solution
The element chlorine is a mixture of
75.77% of 3517Cl and
24.23% of 3717Cl .
So the
average atomic mass will be
(75.77/100) √ó 34.96885u + (24.23/100) √ó 36.96593u
= 35.453u
In fact, the chemist uses the average atomic mass or simply
called chemical atomic weight (35.453 u for chlorine) of an element. So it must
be remembered that the atomic mass which is mentioned in the periodic table is
basically averaged atomic mass.
Related Topics