Nuclear Physics - Mass defect and binding energy | 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Chapter: 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Mass defect and binding energy
Mass defect
and binding energy
It is experimentally found out that
the mass of any nucleus is always less than the sum of the mass of its
individual constituents. For example, consider the carbon-12 nucleus which is
made up of 6 protons and 6 neutrons.
Mass of 6 neutrons = 6×1.00866 u = 6.05196u
Mass of 6 protons = 6 ×1.00727 u = 6.04362 u
Mass of 6 electrons = 6 × 0.00055u = 0.0033u
![]() The
expected mass of carbon-12 nucleus
The
expected mass of carbon-12 nucleus
= 6.05196 u + 6.04362 u = 12.09558u
But using mass spectroscopy, the atomic mass of carbon-12 atom is
found to be 12 u. So if we subtract
the mass of 6 electrons (0.0033 u)
from 12 u, we get the carbon-12
nuclear mass which is equal to 11.9967 u.
Note that the experimental mass of carbon-12 nucleus is less than the total
mass of its individual constituents by ∆m
= 0.09888u . This difference in
mass ∆m is called mass defect. In
general, if M, mp and mn are mass of the nucleus ( AzX ), the mass of a proton and the mass
of a neutron respectively, then the mass defect is given by
∆m = (Zmp + Nmn) - M (8.20)
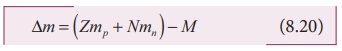
Where has this mass disappeared? The answer was provided by
Albert Einstein with the help of famous mass-energy relation (E = mc2
) . According to this relation, the mass can be converted into energy and
energy can be converted into mass. In the case of the carbon-12 nucleus, when 6
protons and 6 neutrons combine to form carbon-12 nucleus, mass equal to mass
defect disappears and the corresponding energy is released. This is called the
binding energy of the nucleus (BE) and is equal to (∆m)c2 . In fact,
to separate the carbon-12 nucleus into individual constituents, we must supply
the energy equal to binding energy of the nucleus.
We can write the equation (8.20) in terms of binding energy
BE = (Zmp + Nmn + M )c2 (8.21)
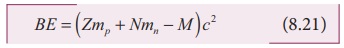
It is always convenient to work with the mass of the atom than
the mass of the nucleus. Hence by adding and subtracting the mass of the Z electrons, we get
BE = (Zmp + Zme + Nmn - M - Zme )c2 (8.22)
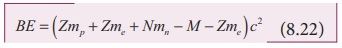
BE = [Z (mp + me ) + Nmn
- M - Zme ] c2
where mp + me = mH (mass of hydrogen atom)
BE = [ZmH + Nmn - (M + Zme) c2 (8.23)
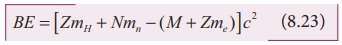
Here M + Zme = MA where MA is the mass
of the atom of an element AZX
.
Finally, the binding energy in terms of the atomic masses is
given by
BE = [ZmH + Nmn - MA ] c2 (8.24)
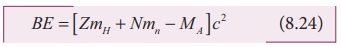
Using Einstein’s mass-energy equivalence, the energy equivalent of
one atomic mass unit 1u = 1.66 ×10-27 × (3 ×108)2
= 14.94 ×10-11
J
≈ 931MeV
EXAMPLE 8.9
Compute the binding energy of 42He nucleus using the following data:
Atomic mass of Helium atom, MA
(He) = 4.00260 u and that of hydrogen atom, mH = 1.00785u.
Solution:
Binding energy BE = [ZmH + Nmn - M A
]c2
For helium nucleus, Z =
2, N = A–Z = 4–2 = 2
Mass defect
∆m = [(2×1.00785u) + (2 ×1.008665u) - 4.00260 u] ∆m = 0.03038u
B.E = 0.03038u × c2
B.E = 0.03038 × 931MeV = 28 MeV
[1uc2 = 931MeV ]
The binding energy of the
42He nucleus
is 28 MeV.
Related Topics