Atom Models | Physics - Rutherford’s model | 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Chapter: 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Rutherford’s model
ATOM MODELS
Rutherford’s
model
In 1911, Geiger and Marsden did a remarkable experiment based on
the advice of their teacher Rutherford, which is known as scattering of alpha
particles by gold foil.
The experimental arrangement is shown in Figure 8.9. A source of
alpha particles (radioactive material, example polonium) is kept inside a thick
lead box with a fine hole as seen in Figure 8.9.
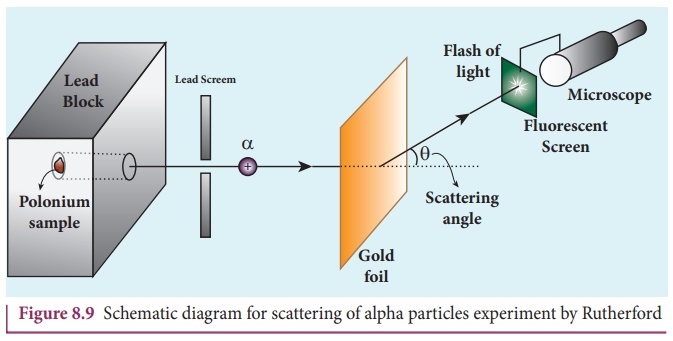
The alpha particles coming through the fine hole of lead box
pass through another fine hole made on the lead screen. These particles are now
allowed to fall on a thin gold foil and it is observed that the alpha particles
passing through gold foil are scattered through different angles. A movable
screen (from 0° to 180°) which is made up of zinc sulphide (ZnS) is kept on the
other side of the gold foil to collect the alpha particles. Whenever alpha
particles strike the screen, a flash of light is observed which can be seen
through a microscope.
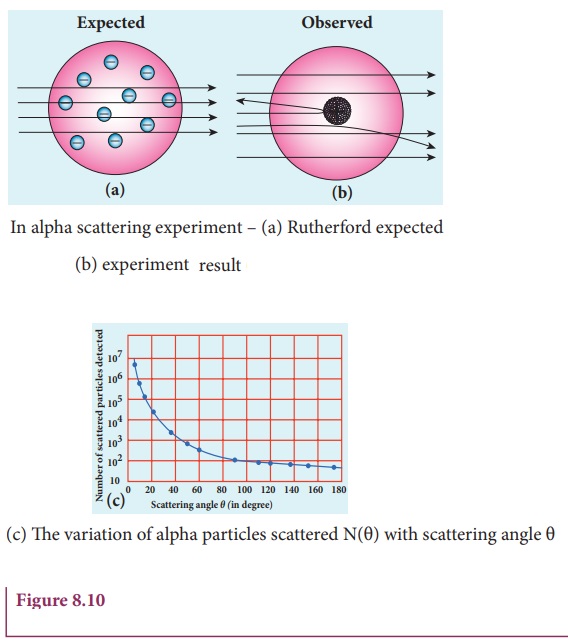
Rutherford proposed an atom model based on the results of alpha scattering experiment. In this experiment, alpha particles (positively charged particles) are allowed to fall on the atoms of a metallic gold foil. The results of this experiment are given below and are shown in Figure 8.10, Rutherford expected the nuclear model to be as seen in Figure 8.10 (a) but the experiment showed the model as in Figure 8.10 (b).
(a) Most of the
alpha particles are un- deflected through the gold foil and went straight.
(b) Some of the alpha particles are deflected through a small angle.
(c) A few alpha particles (one in thousand) are deflected through the angle more than 90°
(d) Very few alpha
particles returned back (back scattered) –that is, deflected back by 180°
In Figure 8.10 (c), the dotted points are the alpha scattering experiment data points obtained by Geiger and Marsden and the solid curve is the prediction from Rutherford’s nuclear model. It is observed that the Rutherford’s nuclear model is in good agreement with the experimental data.
Conclusion made by Rutherford based on the above observation
From the experimental observations, Rutherford proposed that an
atom has a lot of empty space and contains a tiny matter known as nucleus whose
size is of the order of 10-14m. The nucleus is positively charged
and most of the mass of the atom is concentrated in nucleus. The nucleus is
surrounded by negatively charged electrons. Since static charge distribution
cannot be in a stable equilibrium, he suggested that the electrons are not at
rest and they revolve around the nucleus in circular orbits like planets
revolving around the sun.
(a) Distance of closest approach
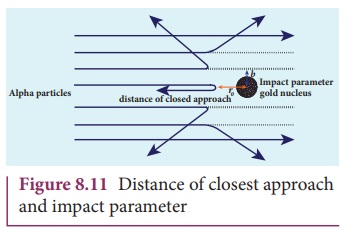
When an alpha particle moves straight towards the nucleus, it reaches a point where it comes to rest momentarily and returns back as shown in Figure 8.11. The minimum distance between the centre of the nucleus and the alpha particle just before it gets reflected back through 180° is defined as the distance of closest approach r0 (also known as contact distance). At this distance, all the kinetic energy of the alpha particle will be converted into electrostatic potential energy (Refer unit 1, volume 1 of +2 physics text book).

where Ek is
the kinetic energy of the alpha particle.This is used to estimate the size of
the nucleus but size of the nucleus is always lesser than the distance of
closest approach. Further, Rutherford calculated the radius of the nucleus for
different nuclei and found that it ranges from 10–14 m to 10–15
m.
(b) Impact parameter
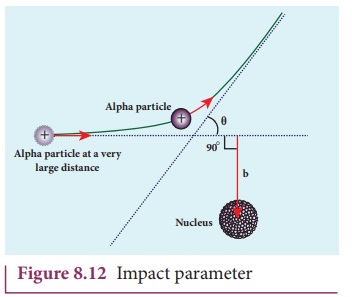
The impact parameter (b) (see Figure 8.12) is defined as the perpendicular distance between the centre of the gold nucleus and the direction of velocity vector of alpha particle when it is at a large distance. The relation between impact parameter and scattering angle can be shown as
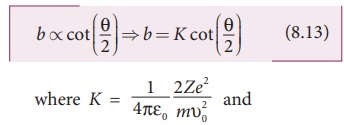
and θ is called scattering angle. Equation (8.13) impliesthat when impact parameter increases, the scattering angle decreases. Smaller the impact parameter, larger will be the deflection of alpha particles.
Drawbacks of Rutherford model
Rutherford atom model helps in the
calculation of the diameter of the nucleus and also the size of the atom but
has the following limitations:
(a) This model fails to explain the distribution of electrons
around the nucleus and also the stability of the atom.
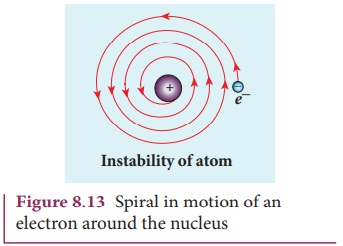
According to classical electrodynamics, any accelerated charge
emits electromagnetic radiations. Due to emission of radiations, it loses its
energy. Hence, it can no longer sustain the circular motion. The radius of the
orbit, therefore, becomes smaller and smaller (undergoes spiral motion) as
shown in Figure 8.13 and finally the electron should fall into the nucleus and
the atoms should disintegrate. But this does not happen.
Hence, Rutherford model could not account for the stability of
atoms.
(b) According to this model, emission of radiation must be continuous
and must give continuous emission spectrum but experimentally we observe only
line (discrete) emission spectrum for atoms.
Related Topics