Nuclear Physics - Size and density of the nucleus | 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Chapter: 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Size and density of the nucleus
Size and
density of the nucleus
The alpha particle scattering experiment and many other measurements
using different methods have been carried out on the nuclei of various atoms.
The nuclei are found to be approximately spherical in shape. It is experimentally
found that radius of nuclei for Z >
10, satisfies the following empirical formula

Here A is the mass
number of the nucleus and the constant R0 = 1.2 F, where 1 F = 1 ×
10–15 m. The unit fermi (F) is named after Enrico Fermi.
EXAMPLE 8.7
Calculate the radius of 19779Au nucleus.
Solution
According to the equation (8.19),
R = 1.2 × 10-15 × (197)1/3
= 6.97 × 10-15m
Or R = 6.97 F
EXAMPLE 8.8
Calculate the density of the nucleus with mass number A.
Solution
From equation (8.19), the radius of the nuclei satisfy the equation
= R0A1/3. Then the volume of the nucleus
V = 4/3 πR3 = 4/3 πR3A
By ignoring the mass difference between the proton and neutron, the total mass of the nucleus having mass number A is equal to A.m where m is mass of the proton and is equal to 1.6726 x 10-27 kg.
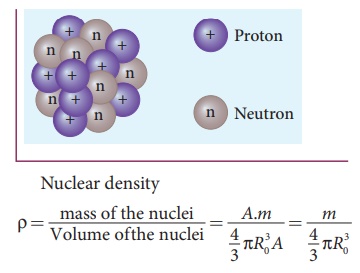
The above expression shows that the nuclear density is
independent of the mass number A. In
other words, all the nuclei (Z >
10) have the same density and it is an important characteristics of the nuclei.
We can calculate the numerical value of this density by
substituting the corresponding values.
It implies that nucleons are extremely tightly packed in the
nucleus and compare this density with the density of water which is 103 kg m-3.
A single teaspoon of nuclear matter would weigh about trillion
tons.
Related Topics