Electric Discharge Through Gases | Physics - Determination of specific charge (e/m) of an electron - Thomson’s experiment | 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Chapter: 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Determination of specific charge (e/m) of an electron - Thomson’s experiment
Determination of specific charge (e/m) of an
electron - Thomson’s experiment
Thomson’s experiment is considered
as one among the landmark experiments for the birth of modern physics. In 1887,
J. J. Thomson made remarkable improvement in the scope of study of gases in
discharge tubes. In the presence of electric and magnetic fields, the cathode
rays are deflected. By the variation of electric and magnetic fields, mass
normalized charge or the specific charge (charge per unit mass) of the cathode
rays is measured.
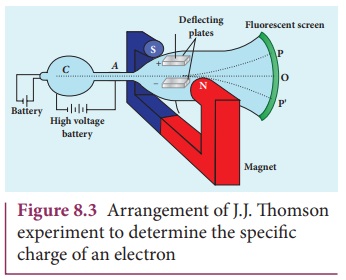
The arrangement of J. J. Thomson’s experiment is shown in Figure 8.3. A highly evacuated discharge tube is used and cathode rays (electron beam) produced at cathode are attracted towards anode disc A. Anode disc is made with pin hole in order to allow only a narrow beam of cathode rays. These cathode rays are now allowed to pass through the parallel metal plates, maintained at high voltage as shown in Figure 8.3. Further, this gas discharge tube is kept in between pole pieces of magnet such that both electric and magnetic fields are perpendicular to each other. When the cathode rays strike the screen, they produce scintillation and hence bright spot is observed. This is achieved by coating the screen with zinc sulphide.
(i) Determination of velocity of cathode rays
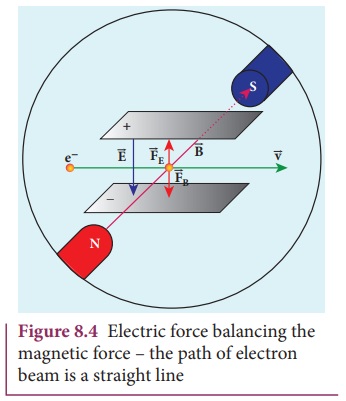
For a fixed electric field between
the plates, the magnetic field is adjusted such that the cathode rays (electron
beam) strike at the original position O (Figure 8.3). This means that the
magnitude of electric force is balanced by the magnitude of force due to
magnetic field as shown in Figure 8.4. Let e
be the charge of the cathode rays, then
eE =eBυ

(ii) Determination of specific charge
Since the cathode rays (electron
beam) are accelerated from cathode to anode, the potential energy of the
electron beam at the cathode is converted into kinetic energy of the electron
beam at the anode. Let V be the
potential difference between anode and cathode, then the potential energy is eV. Then from law of conservation of
energy,
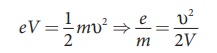
Substituting the value of velocity
from equation (8.1), we get

Substituting the values of E, B and V, the specific charge can be determined as
e/m = 1.7×1011 C kg−1
(iii) Deflection of charge only due to uniform electric field
When the magnetic field is turned
off, the deflection is only due to electric field. The deflection in vertical
direction is due to the electric force.
Fe = eE

Let m be the mass of the electron and by applying Newton’s second law
of motion, acceleration of the electron is

Substituting equation (8.4) in
equation (8.3),
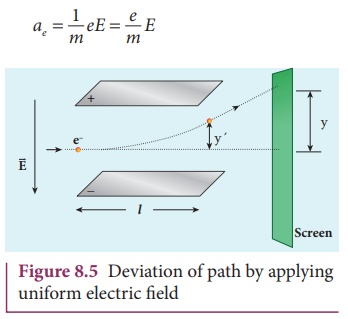
![]() Let y be the deviation produced from
original position on the screen as shown in Figure 8.5. Let the initial upward
velocity of cathode ray be u = 0
before entering the parallel electric plates. Let t be the time taken by the cathode rays to travel in electric
field. Let l be the length of one of
the plates, then the time taken is
Let y be the deviation produced from
original position on the screen as shown in Figure 8.5. Let the initial upward
velocity of cathode ray be u = 0
before entering the parallel electric plates. Let t be the time taken by the cathode rays to travel in electric
field. Let l be the length of one of
the plates, then the time taken is

Hence, the deflection yʹ of cathode rays is (note: u = 0 and ae = e/m E )
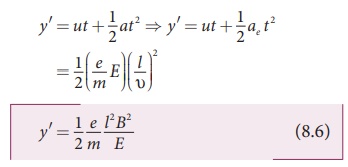
Therefore, the deflection y on the screen is
y ∝ y′
⇒ y = C y′
where C is proportionality constant
which depends on the geometry of the discharge tube and substituting yʹ value in equation 8.6, we get

Rearranging equation (8.7) as

Substituting the values on RHS, the
value of specific charge is calculated as e/m
= 1.7 ×1011C kg−1
(iv) Deflection of charge only due to uniform magnetic field
Suppose that the electric field is
switched off and only the magnetic field is switched on. Now the deflection occurs
only due to magnetic field. The force experienced by the electron in uniform
magnetic field applied perpendicular to its path is
Fm = eυB
(in magnitude)
Since this force provides the
centripetal force, the electron beam undergoes a semi- circular path.
Therefore, we can equate Fm to
centripetal force mυ2 / R.
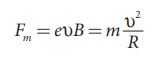
where v is the velocity of electron beam at the point where it enters the
magnetic field and R is the radius of
the circular path traversed by the electron beam.
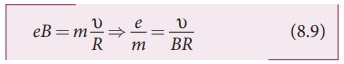
Further, substituting equation (8.1)
in equation (8.10), we get

By knowing the values of electric
field, magnetic field and the radius of circular path, the value of specific
charge (e/m) can be calculated, which is also consistant with other two
methods.
The specific charge is independent
of (a) gas used (b) nature of the electrodes
Related Topics