Chapter: 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Nuclear Fission
NUCLEAR FISSION
In 1939, German scientists Otto Hahn
and F. Strassman discovered that when uranium nucleus is bombarded with a
neutron, it breaks up into two smaller nuclei of comparable masses with the
release of energy. The process of
breaking up of the nucleus of a heavier atom into two smaller nuclei with the
release of a large amount of energy is called nuclear fission. The fission
is accompanied by the release of neutrons. The energy that is released in the
nuclear fission is of many orders of magnitude greater than the energy released
in chemical reactions.
Uranium undergoes fission reaction
in 90 different ways. The most common fission reactions of 23592U nuclei are shown here.
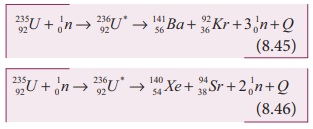
Here Q is energy released during the decay of each uranium nuclei. When
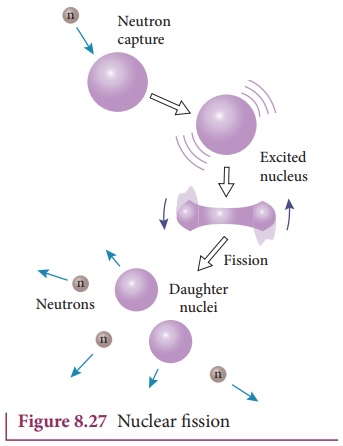
the slow neutron is absorbed by the uranium nuclei, the mass number increases
by one and goes to an excited state 23692U * . But this excited state
does not last longer than 10-12s and decay into two daughter nuclei
along with 2 or 3 neutrons. From each reaction, on an average, 2.5 neutrons are
emitted. It is shown in Figure 8.27
Energy released in fission:
We can calculate the energy (Q) released in each uranium fission
reaction. We choose the most favorable fission which is given in the equation
(8.45).
23592U + 10n → 23692U * → 14156Ba + 9236Kr + 301n + Q
Mass of 23592U = 235.045733 u
Mass of 10n = 1.008665 u
Total mass of reactant = 236.054398 u
Mass of 14156Ba = 140.9177 u
Mass of 9236Kr = 91.8854 u
Mass of 3 neutrons = 3.025995 u
The total mass of products = 235.829095
u
Mass defect ∆m = 236.054398 u –
235.829095 u
![]() = 0.225303 u
= 0.225303 u
So the energy released in each
fission =
0.225303 × 931MeV ≈ 200 MeV
This energy first appears as kinetic
energy of daughter nuclei and neutrons. But later, this kinetic energy is
transferred to the surrounding matter as heat.
Chain reaction:
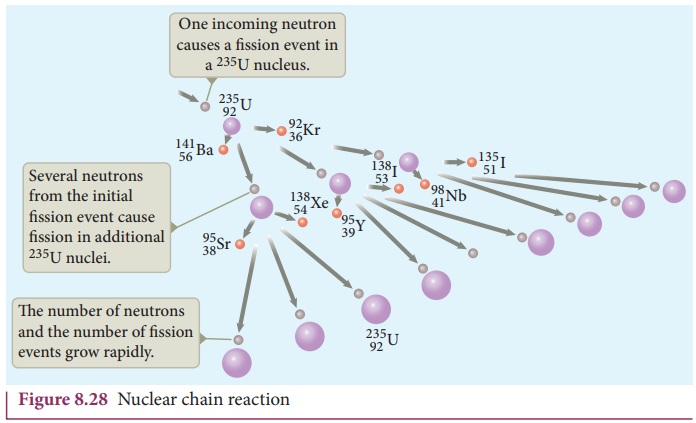
When one 23592U nucleus undergoes fission, the energy
released might be small. But from each fission reaction, three neutrons are
released. These three neutrons cause further fission in another three 23592U nuclei which in turn produce nine
neutrons. These nine neutrons initiate fission in another 27 23592U nuclei and so on. This is called a
chain reaction and the number of neutrons goes on increasing almost in
geometric progression. It is shown in Figure 8.28.
There are two kinds of chain reactions:
(i) uncontrolled chain reaction (ii) controlled chain reaction. In an
uncontrolled chain reaction, the number of neutrons multiply indefinitely and
the entire amount of energy released in a fraction of second.
The atom bomb is an example of nuclear fission in which uncontrolled chain reaction occurs. Atom bombs produce massive destruction for mankind. During World War II, in the year 1946 August 6 and 9, USA dropped two atom bombs in two places of Japan, Hiroshima and Nagasaki. As a result, lakhs of people were killed and the two cities were completely destroyed. Even now the people who are living in those places have side effects caused by the explosion of atom bombs.
It is possible to calculate the
typical energy released in a chain reaction. In the first step, one neutron initiates the fission of
one nucleus by producing three neutrons and energy of about 200 MeV. In the
second step, three nuclei undergo fission, in third step nine nuclei undergo
fission, in fourth step 27 nucleus undergo fission and so on. In the 100th
step, the number of nuclei which undergoes fission is around 2.5 ×1040 .
The total energy released after 100th step is 2.5 ×1040 × 200MeV = 8 ×1029 J . It is really an enormous amount of
energy which is equivalent to electrical energy required in Tamilnadu for
several years.
If the chain reaction is
controllable, then we can harvest an enormous amount of energy for our needs.
It is achieved in a controlled chain reaction. In the controlled chain
reaction, the average number of neutron released in each stage is kept as one
such that it is possible to store the released energy. In nuclear reactors, the
controlled chain reaction is achieved and the produced energy is used for power
generation or for research purpose.
EXAMPLE 8.15
Calculate the amount of energy
released when 1 kg of 93592U undergoes fission reaction.
Solution
235 g of 93592U has
6.02 ×1023 atoms. In one gram
of 93592U , the number of atoms is equal
to 6.02 ×1023 /235 = 2.56 ×1021
.
So the number of atoms in 1 kg of 93592U = 2.56 ×1021 ×1000 = 2.56 ×1024.
Each 93592U nucleus releases 200 MeV of energy during the fission. The total energy released by 1kg of 93592U is
Q = 2.56 ×1024 × 200MeV = 5.12 ×1026 MeV
By converting in terms of joules,
Q = 5.12 ×1026 ×1.6 ×10-13
J = 8.192 ×1013 J .
In terms of Kilowatt hour,
Q = 8.192 ×1013 / 3.6 ×106 = 2.27 ×107 kWh
This is enormously large energy which
is enough to keep 100 W light bulb operating for 30,000 years. To produce this
much energy through chemical reaction, around 20,000 tons of TNT(tri nitro
toluene) has to be exploded.
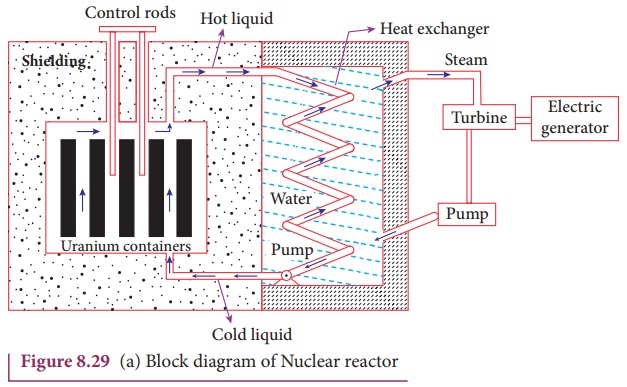
Nuclear reactor:
Nuclear reactor is a system in which
the nuclear fission takes place in a self-sustained controlled manner and the energy
produced is used either for research purpose or for power generation. The first
nuclear reactor was built in the year 1942 at Chicago, USA by physicist Enrico
Fermi. The main parts of a nuclear reactor are fuel, moderator and control
rods. In addition to this, there is a cooling system which is connected with
power generation set up.
Fuel: The fuel is fissionable material, usually uranium or plutonium. Naturally occurring uranium contains only 0.7% of 23592U and 99.3% are only 23892U. So the 23892U must be enriched such that it contains at least 2 to 4% of 23592U . In addition to this, a neutron source is required to initiate the chain reaction for the first time. A mixture of beryllium with plutonium or polonium is used as the neutron source. During fission of 23592U , only fast neutrons are emitted but the probability of initiating fission by it in another nucleus is very low. Therefore, slow neutrons are preferred for sustained nuclear reactions.
Moderators: The moderator is a material
used to convert fast neutrons into slow neutrons. Usually the moderators are
chosen in such a way that it must be very light nucleus having mass comparable
to that of neutrons. Hence, these light nuclei undergo collision with fast
neutrons and the speed of the neutron is reduced (Note that a billiard ball
striking a stationary billiard ball of equal mass would itself be stopped but
the same billiard ball bounces off almost with same speed when it strikes a
heavier mass. This is the reason for using lighter nuclei as moderators). Most
of the reactors use water, heavy water (D2O) and graphite as moderators.
The blocks of uranium stacked together with blocks of graphite (the moderator)
to form a large pile is shown in the Figure 8.29 (a) & (b).
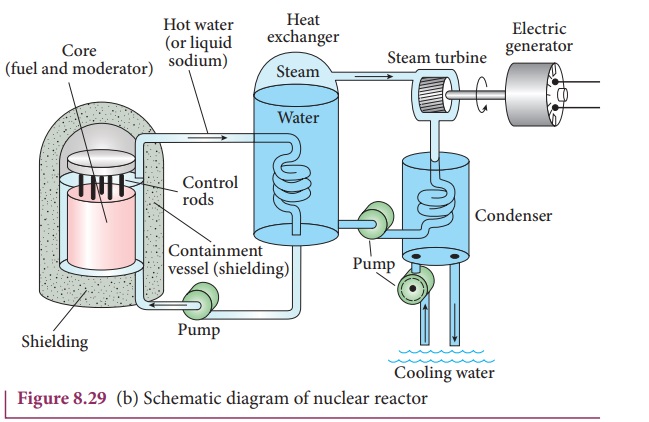
Control rods: The control rods
are used to adjust the reaction rate. During each fission, on an average 2.5
neutrons are emitted and in order to have the controlled chain reactions, only
one neutron is allowed to cause another fission and the remaining neutrons are
absorbed by the control rods.
Usually cadmium or boron acts as
control rod material and these rods are inserted into the uranium blocks as
shown in the Figure 8.29 (a) and (b). Depending on the insertion depth of control
rod into the uranium, the average number of neutrons produced per fission is
set to be equal to one or greater than one. If the average number of neutrons
produced per fission is equal to one, then reactor is said to be in critical
state. In fact, all the nuclear reactors are maintained in critical state by
suitable adjustment of control rods. If it is greater than one, then reactor is
said to be in super-critical and it may explode sooner or may cause massive
destruction.
Shielding: For a protection against
harmful radiations, the nuclear reactor is surrounded by a concrete wall of
thickness of about 2 to 2.5 m.
Cooling system: The cooling system
removes the heat generated in the reactor core. Ordinary water, heavy water and
liquid sodium are used as coolant since they have very high specific heat
capacity and have large boiling point under high pressure. This coolant passes
through the fuel block and carries away the heat to the steam generator through
heat exchanger as shown in Figure 8.29(a) and (b). The steam runs the turbines
which produces electricity in power reactors.
India has 22 nuclear reactors in
operation. Nuclear reactors are constructed in two places in Tamilnadu,
Kalpakkam and Kudankulam. Even though nuclear reactors are aimed to cater to our
energy need, in practice nuclear reactors now are able to provide only 2% of
energy requirement of India.
Related Topics