Nuclear Physics - Radioactivity: Beta decay | 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Chapter: 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Radioactivity: Beta decay
Beta decay
In beta decay, a radioactive nucleus
emits either electron or positron. If electron (e–) is emitted, it is called β- decay and if
positron (e+) is emitted,
it is called β+ decay. The positron is an anti-particle of an
electron whose mass is same as that of electron and charge is opposite to that
of electron – that is, +e. Both
positron and electron are referred to as beta particles.
β− decay:
In β- decay, the atomic number of
the nucleus increases by one but mass number remains the same. This decay is
represented by
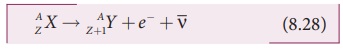
It implies that the element X becomes Y by giving out an electron and antineutrino ( ![]() ). In
otherwords, in each β- decay, one neutron in the nucleus of X is converted into a proton by emitting
an electron (e–) and
antineutrino. It is given by
). In
otherwords, in each β- decay, one neutron in the nucleus of X is converted into a proton by emitting
an electron (e–) and
antineutrino. It is given by
n → p + e− + ![]()
Where p-proton, ![]() -antineutrino.
-antineutrino.
Example: Carbon ( 146C ) is
converted into nitrogen ( 147N ) through β- decay.
146C → 147N + e−
+ ![]()
β + decay:
In β+ decay, the atomic number is
decreased by one and the mass number remains the same. This decay is
represented by
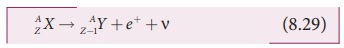
It implies that the element X becomes Y by giving out an positron and neutrino ( υ ). In otherwords, for
each β+ decay, a proton in the nucleus of X is converted into a neutron by emitting a positron (e+) and a neutrino. It is
given by
p → n + e+ + υ
However a single proton (not inside
any nucleus) cannot have β+ decay due to energy conservation,
because neutron mass is larger than proton mass. But a single neutron (not
inside any nucleus) can have β- decay.
A very interesting application of
alpha decay is in smoke detectors which prevent us from any hazardous fire.
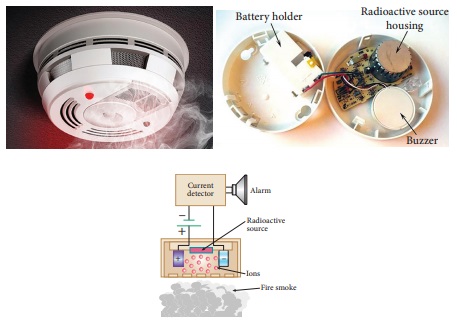
The smoke detector uses around 0.2 mg of man-made weak radioactive isotope
called americium ( 24193Am ). This radioactive source is placed between two oppositely
charged metal plates and α radiations from 24195Am continuously ionize the nitrogen,
oxygen molecules in the air space between the plates. As a result, there will
be a continuous flow of small steady current in the circuit. If smoke enters,
the radiation is being absorbed by the smoke particles rather than air
molecules. As a result, the ionization and along with it the current is
reduced. This drop in current is detected by the circuit and alarm starts.
The radiation dosage emitted by
americium is very much less than safe level, so it can be considered harmless.
Example: Sodium ( 2211Na ) is converted into neon ( 2210Ne ) through β+ decay.
2211Na → 2210Ne + e+
+ υ
It is important to note that the
electron or positron which comes out from nuclei during beta decay never
present inside the nuclei rather they are produced during the conversion of
neutron into proton or proton into neutron inside the nucleus.
Neutrino:
Initially, it was thought that
during beta decay, a neutron in the parent nucleus is converted to the daughter
nuclei by emitting only electron as given by
AzX → z+1AY + e- (8.30)

But the kinetic energy of electron
coming out of the nucleus did not match with the experimental results. In alpha
decay, the alpha particle takes only certain allowed discrete energies whereas
in beta decay, it was found that the beta particle (i.e, electron) have a
continuous range of energies. But the conservation of energy and momentum gives
specific single values for electron energy and the recoiling nucleus Y. It
seems that the conservation of energy, momentum are violated and could not be
explained why energy of beta particle have continuous range of values. So beta
decay remained as a puzzle for several years.
After a detailed theoretical and
experimental study, in 1931 W.Pauli proposed a third particle which must be present
in beta decay to carry away missing energy and momentum. Fermi later named this
particle the neutrino (little neutral
one) since it has no charge, have very little mass. For many years, the
neutrino (symbol υ, Greek nu) was hypothetical
and could not be verified experimentally. Finally, the ![]() neutrino
was detected experimentally in 1956 by Fredrick Reines and Clyde Cowan. Later
Reines received Nobel prize in physics in the year 1995 for his discovery.
neutrino
was detected experimentally in 1956 by Fredrick Reines and Clyde Cowan. Later
Reines received Nobel prize in physics in the year 1995 for his discovery.
The neutrino has the following
properties
• It has zero
charge
• It has an
antiparticle called anti-neutrino.
• Recent
experiments showed that the neutrino has very tiny mass.
• It interacts very weakly with the
matter.
Therefore, it is very difficult to
detect. In fact, in every second, trillions of neutrinos coming from the sun
are passing through our body without any interaction.
Related Topics