Chapter: 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Bohr atom model
ATOM MODEL
Bohr atom
model
In order to overcome the limitations of the Rutherford atom
model in explaining the stability and also the line spectrum observed for a
hydrogen atom (Figure 8.14), Niels Bohr made modifications of Rutherford atom
model. He is the first person to give better theoretical model of the structure
of an atom to explain the line spectrum of hydrogen atom. The following are the
assumptions (postulates) made by Bohr.

Postulates of Bohr atom model:
(a) The electron in
an atom moves around nucleus in circular orbits under the influence of Coulomb
electrostatic force of attraction. This Coulomb force gives necessary
centripetal force for the electron to undergo circular motion.
(b) Electrons in an
atom revolve around the nucleus only in certain discrete orbits called
stationary orbits where it does not radiate electromagnetic energy. Only those
discrete orbits allowed are stable orbits.
The angular momentum of the electron in these stationary orbits
are quantized – that is, it can be written as integer or integral multiple of h / 2π called as reduced Planck’s constant
– that is, h (read it as h-bar) and the
integer n is called as principal quantum number of the orbit.

This condition is known as angular momentum quantization
condition.
According to quantum mechanics, particles like electrons have
dual nature (Refer unit 7, volume 2 of +2 physics text book). The standing wave
pattern of the de Broglie wave associated with orbiting electron in a stable
orbit is shown in Figure 8.15.
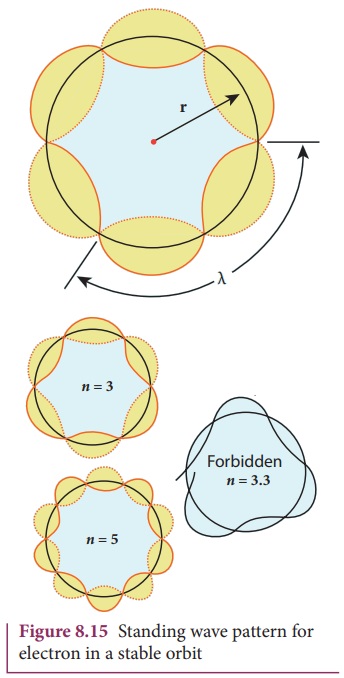
The circumference of an electron’s orbit of radius r must be an integral multiple of de Broglie
wavelength – that is,

But the de Broglie wavelength (λ) for an electron of mass m moving
with velocity υ is λ = h / mυ where h is called Planck’s constant.
Thus from equation (8.14),
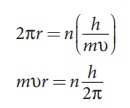
For any particle of mass m
undergoing circular motion with radius r
and velocity υ, the magnitude of
angular momentum l is given by
l = r(mυ)
mυr
= l = nh
(c) Energy of orbits are not continuous but discrete. This is
called the quantization of energy. An electron can jump from one orbit to
another orbit by absorbing or emitting a photon whose energy is equal to the
difference in energy (ΔE) between the two orbital levels (Figure 8.16)
∆E = E final − Einitial = hv = h c/λ
where c is the speed
of light and λ is the wavelength of the radiation used and v is the frequency of the radiation. Thus, the frequency of the
radiation emitted is related only to change in atom’s energy and it does not
depend on frequency of electron’s orbital motion.
EXAMPLE 8.1
The radius of the 5th orbit of
hydrogen atom is 13.25 Å. Calculate the wavelength of the electron in the 5th
orbit.
Solution:
2πr = nλ
2 × 3.14 × 13.25Å = 5 × λ
λ = 16.64Å
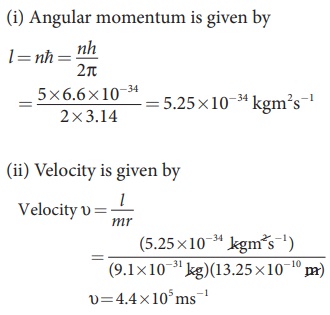
EXAMPLE 8.2
Find the (i)
angular momentum (ii) velocity of the
electron in the 5th orbit of hydrogen atom. (h = 6.6 × 10–34 Js, m = 9.1 × 10–31 kg)
Solution
Radius of the orbit of the electron and velocity of the electron
Consider an atom which contains the nucleus at rest and an
electron revolving around the nucleus in a circular orbit of radius rn as shown in Figure 8.17. Nucleus
is made up of protons and neutrons. Since proton is positively charged and
neutron is electrically neutral, the charge of a nucleus is purely the total
charge of protons.
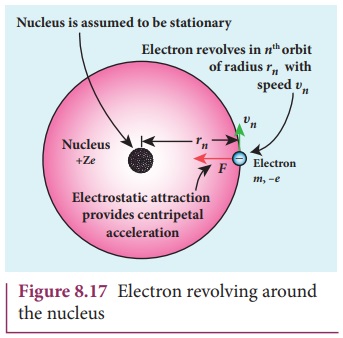
Let Z be the atomic
number of the atom, then +Ze is the
charge of the nucleus. Let –e be the
charge of the electron. From Coulomb’s law, the force of attraction between the
nucleus and the electron is
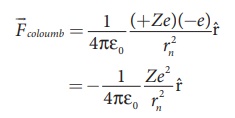
This force provides necessary centripetal force
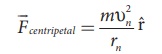
where m be the mass of
the electron that moves with a velocity υn
in a circular orbit. Therefore,
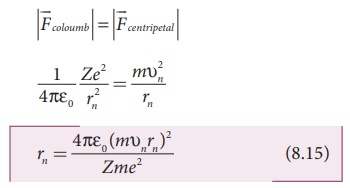
From Bohr’s assumption, the angular momentum quantization condition,
mυnrn
= ln = nh,
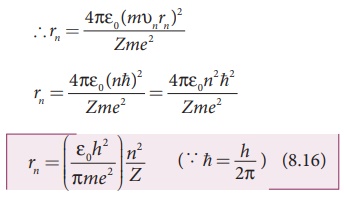
where n∈N . Since, ε0, h,
e and π are constants. Therefore, the radius of the orbit becomes
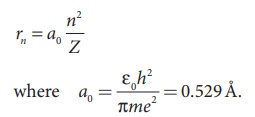
This is known as Bohr radius which is the smallest radius of the
orbit in an atom. Bohr radius is also used as unit of length called Bohr. 1
Bohr = 0.53 Å. For hydrogen atom (Z = 1), the radius of nth orbit is
rn = a0n2
For n = 1 (first orbit
or ground state),
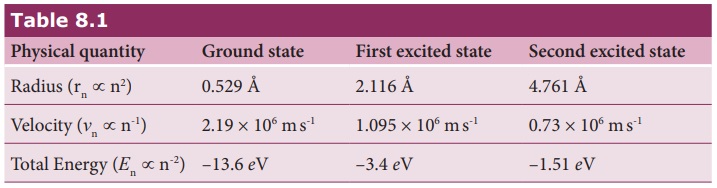
r1 = a0 = 0.529 Å
For n = 2 (second orbit or first excited state),
r2 = 4a0 = 2.116 Å
For n = 3 (third orbit or second excited state),
r3 = 9a0 = 4.761 Å
and so on.
Thus the radius of the orbit from centre increases with n, that is, rn ∝ n2
as shown in Figure 8.18.
Further, Bohr’s angular momentum quantization condition leads to
mυnrn
= mυna0n2 = n h/2π
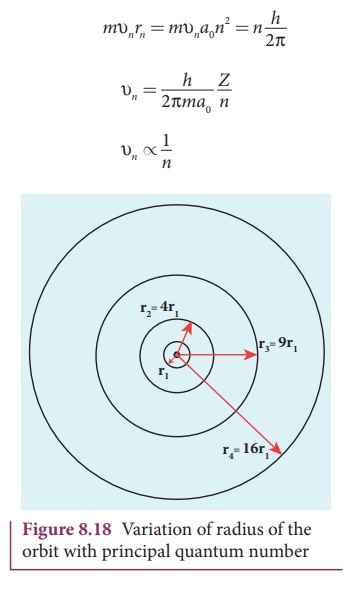
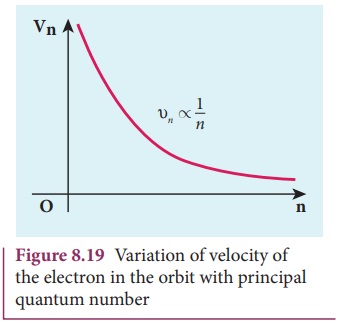
Note that the velocity of electron decreases as the principal
quantum number increases as shown in Figure 8.19. This curve is the rectangular
hyperbola. This implies that the velocity of electron in ground state is
maximum when compared to excited states.
The energy of an electron in the nth orbit
Since the electrostatic force is a conservative force, the
potential energy for the nth orbit is
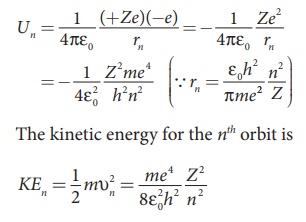
This implies that Un = –2 KEn. Total energy
in the nth orbit is
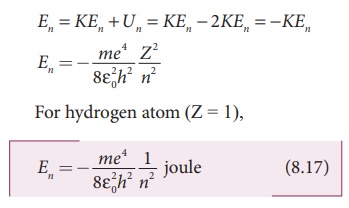
where n stands for
principal quantum number. The negative sign in equation (8.17) indicates that
the electron is bound to the nucleus.
Substituting the values of mass and
charge of an electron (m and e), permittivity of free space ε0
and Planck’s constant h and
expressing in terms of eV, we get
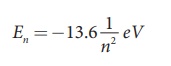
For the first orbit (ground state),
the total energy of electron is E1= – 13.6 eV.
For the second orbit (first excited
state), the total energy of electron is E2 = –3.4 eV. For the third orbit (second excited
state), the total energy of electron is E3 = –1.51 eV and so on.
Notice that the energy of the first excited state is greater
than the ground state, second excited state is greater than the first excited
state and so on. Thus, the orbit which is closest to the nucleus (r1) has lowest energy (minimum
energy compared with other orbits). So, it is often called ground state energy
(lowest energy state). The ground state energy of hydrogen (–13.6 eV ) is used as a unit of energy called
Rydberg (1 Rydberg = –13.6 eV ).
The negative value of this energy is because of the way the zero
of the potential energy is defined. When the electron is taken away to an
infinite distance (very far distance) from nucleus, both the potential energy
and kinetic energy terms vanish and hence the total energy also vanishes.
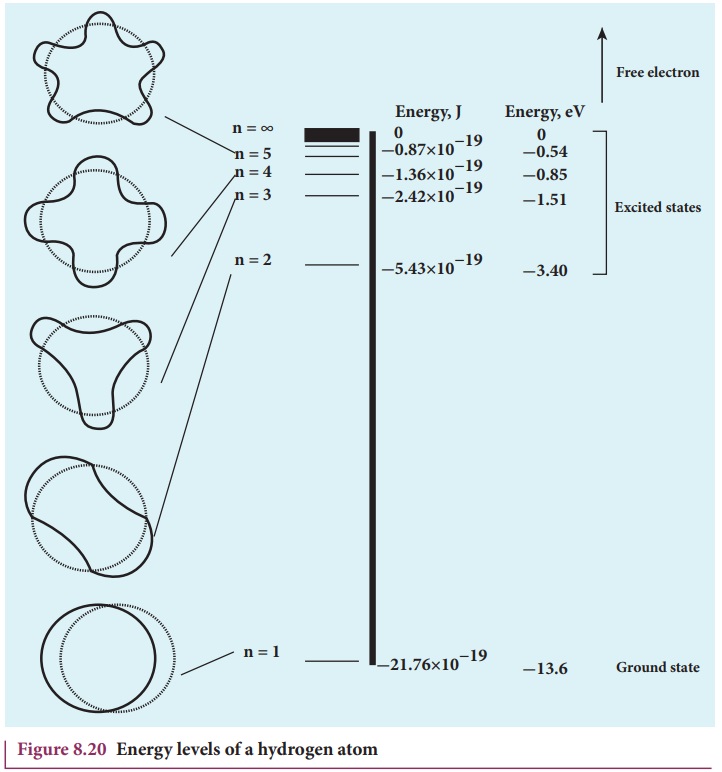
The energy level diagram along with the shape of the orbits for
increasing values of n are shown in
Figure 8.20. It shows that the energies of the excited states come closer and
closer together when the principal quantum number n takes higher values.
EXAMPLE 8.3
(a) Show that the ratio of velocity of an electron in the first
Bohr orbit to the speed of light c is
a dimensionless number.
(b) Compute the velocity of electrons in ground state, first
excited state and second excited state in Bohr atom model.
Solution
(a) The velocity of an electron in nth orbit is
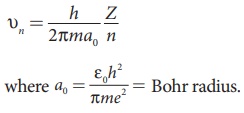
Substituting for a0
in υn,
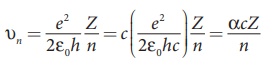
where c is the speed
of light in free space or vacuum and its value is c = 3 × 108 m s–1and α is called fine
structure constant.
For a hydrogen atom, Z =
1 and for the first orbit, n = 1, the
ratio of velocity of electron in first orbit to the speed of light in vacuum or
free space is
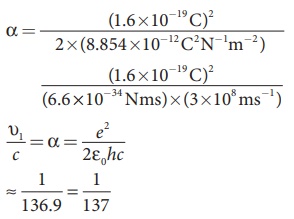
which is a dimensionless number
⇒ α = 1/137
(b) Using fine structure constant,
the velocity of electron can be written as
υn
= αcZ / n
For hydrogen atom (Z = 1) the velocity of electron in nth orbit is
υn = c/137 × 1/n = (2.19×106) × 1/n ms−1
For the first orbit (ground state),
the velocity of electron is
υ = 2.19×106 ms−1
For the second orbit (first excited
state), the velocity of electron is
υ2 = 1.095×106 ms−1
For the third orbit (second excited
state), the velocity of electron is
υ3 = 0.73×106 ms−1
Here, υ1 > υ2
> υ3
EXAMPLE 8.4
The Bohr atom model is derived with the assumption that the
nucleus of the atom is stationary and only electrons revolve around the
nucleus. Suppose the nucleus is also in motion, then calculate the energy of this
new system.
Solution
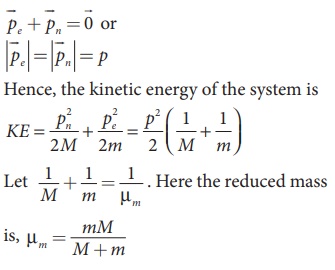
Let the mass of the electron be m and mass of the nucleus be M.
Since there is no external force acting on the system, the centre of mass of
hydrogen atom remains at rest. Hence, both nucleus and electron move about the
centre of mass as shown in figure.
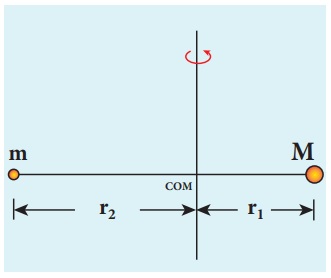
Let V be the velocity
of the nuclear motion and υ be the
velocity of electron motion. Since the total linear momentum of the system is
zero,
−mυ + Mυ = 0 or
MV = mυ = p
Since the potential energy of the system is same, the total
energy of the hydrogen can be expressed by replacing mass by reduced mass,
which is
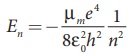
Since the nucleus is very heavy compared to the electron, the
reduced mass is closer to the mass of the electron.
In 1931, H.C. Urey and co- workers
noticed that in the shorter wavelength region of the hydrogen spectrum lines,
faint companion lines are observed. From the isotope displacement effect
(isotope shift), the isotope of the same element will have slightly different
spectral lines. The presence of these faint lines confirmed the existence of
isotopes of hydrogen atom (which is named as Deuterium).
On calculating wavelength or wave
number difference between the faint and bright spectral lines, atomic mass of
deuterium is measured to be twice that of atomic mass of hydrogen atom. Bohr
atom model could not explain this isotopic shift. Thus by considering nuclear
motion (although the movement of the nucleus is much smaller, it is observed)
into account in the Bohr atom model, the wave number or wavelength difference
between hydrogen atom and deuterium is theoretically calculated which perfectly
agreed with the spectroscopic measured values.
The difference between hydrogen atom
and deuterium is in the number of neutron. Hydrogen atom contains an electron
and a proton, whereas deuterium has an electron, a proton and a neutron.
Excitation energy and
excitation potential
The energy required to excite
an electron from lower energy state to any higher energy state is known as
excitation energy.
The excitation energy for an electron from ground state (n = 1)
to first excited state (n = 2) is called first excitation energy, which is
EI = E2 – E1 = –3.4 eV – (–13.6 eV) = 10.2 eV
Similarly, the excitation energy for an electron from ground
state (n = 1) to second excited state (n = 3) is called second excitation
energy, which is
Eu = E3 – E1 = –1.51 eV – (–13.6 eV) = 12.1 eV
and so on.
Excitation potential is defined as excitation energy per unit charge.
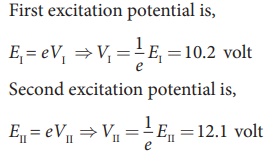
and so on.
Ionization energy and
ionization potential
An atom is said to be ionized when an electron is completely
removed from the atom – that is, it reaches the state with energy En →∞. The minimum energy
required to remove an electron from an atom in the ground state is known as
binding energy or ionization energy.
Eionizaation = E∞ - E1 = 0 -
(13.6eV)
= 13.6 eV
When an electron is in nth state of an atom, the
energy spent to remove an electron from that state – that is, its ionization
energy is
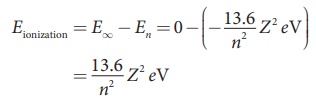
At normal room temperature, the electron in a hydrogen atom (Z=1) spends most of its time in the ground state. The amount of energy spent to remove an electron from the ground state of an atom (E = 0 for n→∞) is known as first ionization energy (13.6 eV).
Then, the hydrogen atom is said to be in ionized state or simply
called as hydrogen ion, denoted by H+.
If we supply more energy than the ionization energy, the excess energy will be
the kinetic energy of the free electron.
Ionization potential is defined
as ionization energy per unit charge.
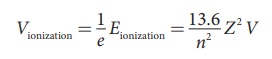
Thus, for a hydrogen atom (Z =1), the ionization potential is
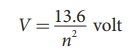
The radius, velocity and total energy in ground state, first
excited state and second excited state is listed in Table 8.1.
EXAMPLE 8.5
Suppose the energy of a hydrogen–like atom is given as En = − 54.4/n2 eV where n∈N . Calculate the following:
(a) Sketch the
energy levels for this atom and compute its atomic number.
(b) If the atom is in ground state, compute its first excitation
potential and also its ionization potential.
(c) When a photon with energy 42 eV and another photon with energy 56 eV are made to collide with this atom, does this atom absorb these
photons?
(d) Determine the radius of its first Bohr orbit.
(e) Calculate the kinetic and potential energies in the ground
state.
Solutions
(a) Given that
En =− 54.4/n2 eV
For n = 1, the ground
state energy E1= –54.4 eV
and for n = 2, E2 = –13.6 eV.
Similarly, E3 = –6.04 eV, E4 = –3.4 eV
and so on.
For large value of principal quantum number – that is, n = ∞, we get E∞ = 0 eV.

(b) For a hydrogen-like atom, ground state energy is
E1 = − 13.6/n2 Z2 eV
where Z is the atomic
number. Hence, comparing this energy with given energy, we get, – 13.6 Z2 = – 54.4 ⇒ Z = ±2. Since, atomic
number cannot be negative number, Z =
2.
(c) The first excitation energy is
EI = E2 − E1
= −13.6eV −(−54.4 eV)
= 40.8eV
Hence, the first excitation potential is
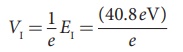
= 40.8 volt
The first ionization energy is
Eionization = E∞ − E1
= 0 −(−54.4 eV)
= 54.4 eV
Hence, the first ionization potential is
Vionization = 1/e Eionization = (54.4eV)/e
= 54.4 Volt
(d) Consider two photons to be A and B.
Given that photon A with energy 42 eV and photon B with energy 51 eV
From Bohr assumption, difference in energy levels is equal to
photon energy, then atom will absorb energy, otherwise, not.
E2 − E1 =
−13.6eV −(−54.4 eV)
= 40.8eV ≈ 41eV
Similarly,
E3 − E1 =
−6.04 eV −(−54.4 eV)
= 48.36eV
E4 − E1 =
−3.4 eV −(−54.4 eV)
= 51eV
E3 − E2 =
−6.04 eV −(−13.6eV)
= 7.56eV
and so on.
But note that E2 – E1 ≠ 42 eV, E3 – E1
≠ 42 eV, E4 – E1 ≠ 42 eV and E3 – E2
≠ 42 eV.
For all possibilities, no difference
in energy is an integer multiple of photon energy. Hence, photon A is not
absorbed by this atom. But for Photon B, E4
– E1 = 51 eV, which
means, Photon B can be absorbed by this atom.
(e) Since total energy is equal to
negative of kinetic energy in Bohr atom model, we get
KEn = −En = −( − 54.4/n2 eV)
= 54.4/n2
eV
Potential energy is negative of
twice the kinetic energy, which means,
Un = −2KEn = −2(54.4/n2 eV)
= −108.8/n2 eV
For a ground state, put n =1
Kinetic energy is KE1 =
54.4 eV and Potential energy is U1 = –108.8 eV
Related Topics