Nuclear Physics - Radioactivity: Alpha decay | 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Chapter: 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Radioactivity: Alpha decay
Alpha decay
When unstable nuclei decay by
emitting an α-particle ( 42He nucleus), it loses two protons and two neutrons. As a result,
its atomic number Z decreases by 2, the mass number decreases by 4. We write
the alpha decay process symbolically in the following way

Here X is called the parent nucleus and Y is called the daughter nucleus.
Example: Decay of Uranium 23892U to thorium 23490Th with the emission of 42He nucleus (α-particle)
23892U → 23490Th + 42He
As already mentioned, the total mass
of the daughter nucleus and 42He nucleus is always less
than that of the parent nucleus. The difference in mass ( ∆m = mX − mY − mα ) is released as energy called disintegration energy Q and
is given by
Q = (mX − mY
− mα )c2 (8.27)
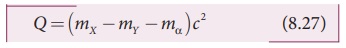
Note that for spontaneous decay
(natural radioactivity) Q >0. In
alpha decay process, the disintegration energy is certainly positive (Q > 0). In fact, the disintegration
energy Q is also the net kinetic
energy gained in the decay process or if the parent nucleus is at rest, Q is the total kinetic energy of
daughter nucleus and the 42He nucleus. Suppose Q <
0, then the decay process cannot occur spontaneously and energy must be
supplied to induce the decay.
In alpha decay, why does the
unstable nucleus emit 42He nucleus? Why it does not emit
four separate nucleons? After all He consists of two protons and two neutrons. For
example, if 92238U nucleus decays into 90234Th
by emitting four separate nucleons (two protons and two neutrons), then the
disintegration energy Q for this process turns out to be negative. It implies
that the total mass of products is greater than that of parent( 92238U
) nucleus.
This kind of process cannot occur in
nature because it would violate conservation of energy. In any decay process,
the conservation of energy, conservation of linear momentum and conservation of
angular momentum must be obeyed.
EXAMPLE 8.11
(a) Calculate the disintegration energy when stationary
23292U nucleus decays to thorium 22890Th
with the emission of α particle. The atomic masses are of 23292U
= 232.037156 u , 22890Th = 228.028741u and 42He
= 4.002603u
(b) Calculate kinetic energies of 22890Th and
α-particle and their ratio.
Solution
The difference in masses
∆m
= (mU – mTh - mα)
= (232.037156–228.028741 –
4.002603)u
The mass lost in this decay =
0.005812 u
Since 1u = 931MeV, the energy Q
released is
Q = (0.005812u)×(931MeV / u)
= 5.41MeV
This disintegration energy Q appears
as the kinetic energy of α particle and the daughter nucleus.
In any decay, the total linear
momentum must be conserved.
Total linear momentum of the parent
nucleus = total linear momentum of the daughter nucleus +α particle
Since before decay, the uranium
nucleus is at rest, its momentum is zero.
By applying conservation of
momentum, we get
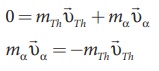
It implies that the alpha particle
and daughter nucleus move in opposite directions.
In magnitude mα υα = mThυTh
The velocity α particle υα
= [mTh/mα]
υTh.
Note that mTh /ma
> 1, so υα > υTh . The ratio of the kinetic energy
of α particle to the daughter nucleus
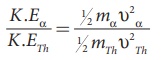
By substituting, the value of υα
into the above equation, we get
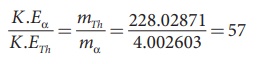
The kinetic energy of α particle is
57 times greater than the kinetic energy of the daughter nucleus (22890Th ).
The disintegration energy Q = total
kinetic energy of products
K.Eα
+ K.ETh = 5.41MeV
57K.ETh + K.ETh
= 5.41MeV
K.ETh
= 5.41/58 MeV = 0.093MeV
K.Eα
= 57K.ETh = 57×0.093 = 5.301MeV
In fact, 98% of total kinetic energy
is taken by the α particle.
Related Topics