Radioactivity | Nuclear Physics - Half-life | 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Chapter: 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Half-life
Half-life
It is difficult to calculate the
time taken by a given a sample of N atoms to decay. However, we can calculate
the time taken by the given sample of atoms to reduce some fraction of the
initial amount.
We can define the half-life T1/2 as the time required for
the number of atoms initially present to reduce to one half of the initial
amount.
The half-life is the important
characteristic of every radioactive sample. Some radioactive nuclei are known
to have half-life as long as 1014 years and some nucleus have very
shorter life time (10-14s).
We can express half-life in terms of
the decay constant. At t = T1/2 , the number of
undecayed nuclei N = N0/2 .
By substituting this value in to the
equation (8.35), we get
N0/2 = N0 e−λT1/2
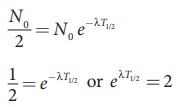
Taking logarithm on both sides and
rearranging the terms,

One should not think that shorter half-life
material is safer than longer half-life material because it will not last long.
The shorter half-life sample will have higher activity and it is more
‘radioactive’ which is more harmful.
If the number of atoms present at
t=0 is N0, then N0/2
atoms remain undecayed in first half-life and N0/4 atoms remain undecayed after second half life and
so on. In general, after n half-lives,
the number of nuclei remaining undecayed is given by

where n can be integer or non-integer.
Since the activity of radioactive
sample also obeys the exponential decay law, we can also write an equation for
an activity similar to equation (8.36).
After n half-lives, the activity or decay rate of any radioactive sample
is

Mean life (τ):
When the radioactive nucleus undergo
the decay, the nucleus which disintegrates first has zero life time and the
nucleus which decay last has an infinite lifetime. The actual life time for
each nucleus varies from zero to infinity. Therefore, it is meaningful to
define average life or mean life time τ, that the nucleus survives before it
decays.
The mean life time of the nucleus is the ratio of sum or
integration of life times of all nuclei to the total number nuclei present
initially.
The total number of nuclei decaying
in the time interval from t to t + ∆t
is equal to R∆t = λN0
e−λt ∆t.
It implies that until the time t,
this R∆t number of nuclei lived. So the life time of these R∆t
nuclei is equal to be tR∆t . In the limit ∆t → 0 , the total life time of all the nuclei would be the
integration of tRdt from the limit t = 0 to t = ∞ .
Mean life
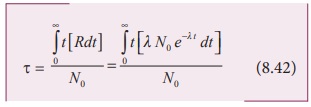
After a few integration (refer box
item), the expression for mean life time,
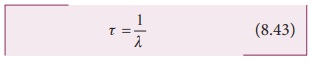
Note that mean life and decay
constant is inversely proportional to each other.
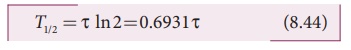
Using mean life, the half-life can
be rewritten as
T1/2 = τ ln 2 = 0.6931τ (8.44)
Mean life : Not for examination
The integration in the equation
(8.42) can be performed using integration by parts.
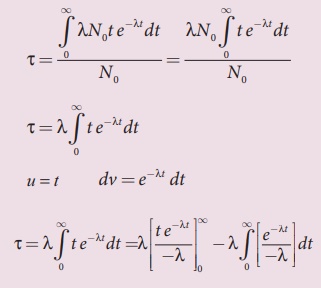
By substituting the limits, the
first term in the above equation becomes zero.
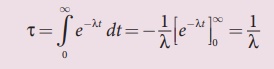
EXAMPLE 8.12
Calculate the number of nuclei of
carbon-14 undecayed after 22,920 years if the initial number of carbon-14 atoms
is 10,000. The half-life of carbon-14 is 5730 years.
Solution
To get the time interval in terms of
half- life,
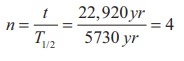
The number of nuclei remaining
undecayed after 22,920 years,
N = ( ½ )0 N0 = (1/2)4 ×10,
000
N = 625
EXAMPLE 8.13
A radioactive sample has 2.6 μg of pure 137N which has a half-life of 10 minutes
(a) How many nuclei are present initially? (b) What is the activity initially?
(c) What is the activity after 2 hours? (d) Calculate mean life of this sample.
Solution
(a) To find N0, we have to find the number
of 137N atoms in 2.6μg . The atomic mass of nitrogen is
13. Therefore, 13 g of 137N contains Avogadro number (
6.02 ×1023 ) of atoms.
In 1 g, the number of 137N
is equal to be 6.02×1023 / 13 atoms. So the number of 137N
atoms in 2.6μg is
N = (6.02×1023/13) × 2.6×10−6
= 12.04×1016 atoms
(b) To find the initial activity R0, we
have to evaluate decay constant λ
λ = 0.6931 / T1/2 = 0.6931 / 10×60 =
1.155×10−3 s−1
Therefore
R0 = λN0 =
1.155×10-3 ×12.04×1016
=13.90×1013 decays/s
= 13.90×1013 Bq
In terms of a curie,
R0 = 13.90×1015
/ 3.7×1010 = 3.75×103 Ci
since 1Ci = 3.7×1010Bq
(c) Activity after 2 hours can be calculated in two different
ways:
Method 1: R = R0e–λt
At t = 2 hr = 7200 s
R = 3.75 × 103 × e–7200 ×1.155 ×10–3
R = 3.75 × 103 × 2.4 ×10–4
= 0.9 Ci
Method 2: R = (1/2)n R0
Here n = 120 min /10 min = 12
R = (1/2)12 ×3.75×103
≈ 0.9Ci
(d) mean life τ = T1/2 / 0.6931 = 10×60 /
0.6931
= 865.67 s
Related Topics