Electric Discharge Through Gases | Physics - Determination of charge of an electron - Millikan’s oil drop experiment | 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Chapter: 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Determination of charge of an electron - Millikan’s oil drop experiment
Determination
of charge of an electron – Millikan’s oil drop experiment
Millikan’s oil drop experiment is another important experiment
in modern physics which is used to determine one of the fundamental constants
of nature known as charge of an electron (Figure 8.6 (a)).
By adjusting electric field
suitably, the motion of oil drop inside the chamber can be controlled – that
is, it can be made to move up or down or even kept balanced in the field of
view for sufficiently long time.
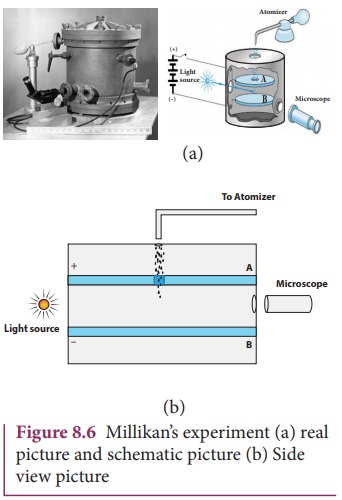
The experimental arrangement is shown in Figure 8.6 (b). The
apparatus consists of two horizontal circular metal plates A and B each with
diameter around 20 cm and are separated by a small distance 1.5 cm.
These two parallel plates are enclosed in a chamber with glass
walls. Further, plates A and B are given a high potential difference around 10 kV such that electric field acts
vertically downward. A small hole is made at the centre of the upper plate A
and atomizer is kept exactly above the hole to spray the liquid. When a fine droplet
of highly viscous liquid (like glycerine) is sprayed using atomizer, it falls
freely downward through the hole of the top plate only under the influence of
gravity.
Few oil drops in the chamber can acquire electric charge
(negative charge) because of friction with air or passage of x-rays in between
the parallel plates. Further the chamber is illuminated by light which is
passed horizontally and oil drops can be seen clearly using microscope placed
perpendicular to the light beam. These drops can move either upwards or
downward.
Let m be the mass of the oil drop and q be its charge. Then the forces acting on the droplet are
(a) gravitational
force Fg = mg
(b) electric force Fe = qE
(c) buoyant force Fb
(d) Viscous force Fv
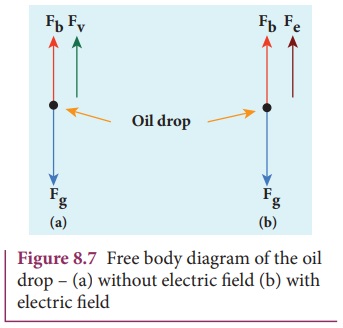
(a) Determination of radius of the droplet
When the electric field is switched off, the oil drop
accelerates downwards. Due to the presence of air drag forces, the oil drops
easily attain its terminal velocity and moves with constant velocity. This
velocity can be carefully measured by noting down the time taken by the oil
drop to fall through a predetermined distance. The free body diagram of the oil
drop is shown in Figure 8.7 (a), we note that viscous force and buoyant force
balance the gravitational force.
Let the gravitational force acting on the oil drop (downward) be
Fg = mg
Let us assume that oil drop to be
spherical in shape. Let ρ be the density of the oil drop, and r be the radius of the oil drop, then
the mass of the oil drop can be expressed in terms of its density as
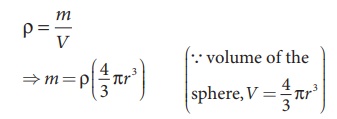
The gravitational force can be written in terms of density as
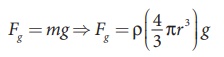
Let σ be the density of the air, the upthrust force experienced
by the oil drop due to displaced air is

Once the oil drop attains a terminal velocity υ, the net downward force acting on the
oil drop is equal to the viscous force acting opposite to the direction of
motion of the oil drop. From Stokes law, the viscous force on the oil drop is
Fv =6πrυη
From the free body diagram as shown in Figure 8.7 (a), the force
balancing equation is

Thus, equation (8.11) gives the radius of the oil drop.
(b) Determination of electric charge
When the electric field is switched on, charged oil drops
experience an upward electric force (qE).
Among many drops, one particular drop can be chosen in the field of view of microscope
and strength of the electric field is adjusted to make that particular drop to
be stationary. Under these circumstances, there will be no viscous force acting
on the oil drop. Then, from the free body diagram shown Figure 8.7 (b), the net
force acting on the oil droplet is
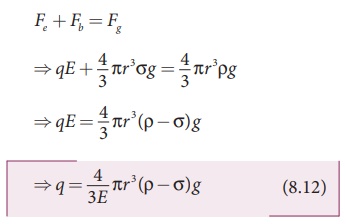
Substituting equation (8.11) in equation (8.12), we get
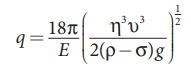
Millikan repeated this experiment several times and computed the
charges on oil drops. He found that the charge of any oil drop can be written
as integral multiple of a basic value, −1.6×10−19C, which is nothing
but the charge of an electron.
Related Topics