Atom Models | Physics - J. J. Thomson’s Model (Water melon model) | 12th Physics : UNIT 9 : Atomic and Nuclear Physics
Chapter: 12th Physics : UNIT 9 : Atomic and Nuclear Physics
J. J. Thomson’s Model (Water melon model)
ATOM MODELS
J. J.
Thomson’s Model (Water melon model)
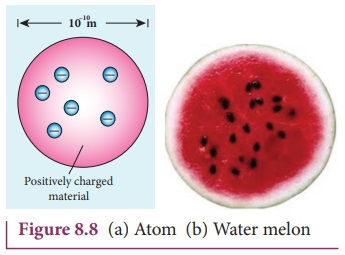
In this model, the atoms are visualized as homogeneous spheres
which contain uniform distribution of positively charged particles (Figure 8.8
(a)). The negatively charged particles known as electrons are embedded in it
like seeds in water melon as shown in Figure 8.8 (b).
The atoms are electrically neutral, this implies that the total
positive charge in an atom is equal to the total negative charge. According to
this model, all the charges are assumed to be at rest. But from classical
electrodynamics, no stable equilibrium points exist in electrostatic
configuration (this is known as Earnshaw’s theorem) and hence such an atom
cannot be stable. Further, it fails to explain the origin of spectral lines
observed in the spectrum of hydrogen atom and other atoms.
Related Topics