Chapter: 11th Chemistry : UNIT 10 : Chemical bonding
Chemical bonding: Introduction
Chemical
bonding
Introduction
Diamond
is very hard while its allotrope graphite is very soft. Gases like hydrogen and
oxygen are diatomic while the inert gases are monoatomic.
Carbon
combines with chlorine to form carbon tetrachloride, which is a liquid and
insoluble (immiscible) in water. Sodium combines with chlorine atom to form
sodium chloride, a hard and brittle compound that readily dissolves in water.
The possible reason for these observations lies in the type of interaction that
exists between the atoms of these molecules and these interactions are
responsible for holding the atoms/ions together. The interatomic attractive
forces which hold the constituent atoms/ions together in a molecule are called
chemical bonds.
Why
do atoms combine only in certain combinations to form molecules? For example
oxygen combines with hydrogen to give water (H2O) and with carbon it
gives carbon dioxide (CO2). The structure of water is ‘V’ shaped
while that of the carbon dioxide is linear. Such questions can be answered
using the principles of chemical bonding. In this unit we will analyse the
various theories and their principles, which were developed over the years to
explain the nature of chemical bonding.
Kossel – Lewis approach to chemical bonding
A
logical explanation for chemical bonding was provided by Kossel and Lewis in
1916. Their approach to chemical bonding is based on the inertness of the noble
gases which have little or no tendency to combine with other atoms. They
proposed that the noble gases are stable due to their completely filled outer
shell electronic configuration. Elements other than noble gases, try to attain
the completely filled electronic configurations by losing, gaining or sharing
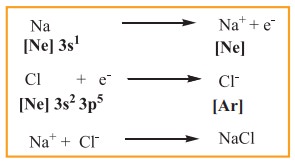
one or more electrons from their outer shell. For example, sodium loses one
electron to form Na+ ion and chlorine accepts that electron to give
chloride ion (Cl–), enabling both atoms to attain the nearest noble
gas configuration. The resultant ions, Na+ and Cl- are
held together by electrostatic attractive forces and the attractive force is
called a chemical bond, more specifically an electrovalent bond.
G.
N. Lewis proposed that the attainment of stable electronic configuration in
molecules such as diatomic nitrogen, oxygen etc… is achieved by mutual sharing
of the electrons. He introduced a simple scheme to represent the chemical bond
and the electrons present in the outer shell of an atom, called Lewis dot
structure. In this scheme, the valence electrons (outer shell electrons) of an
element are represented as small dots around the symbol of the element. The
first four valence electrons are denoted as single dots around the four sides
of the atomic symbol and then the fifth onwards, the electrons are denoted as
pairs. For example, the electronic configuration of nitrogen is 1s2,
2s2, 2p3. It
has 5 electrons in its outer shell (valence shell). The Lewis structure of
nitrogen is as follows.

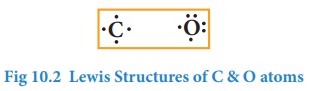
Similarly,
Lewis dot structure of carbon, oxygen can be drawn as shown below.
Only
exception to this is helium which has only two electrons in its valence shell
which is represented as a pair of dots (duet).

Octet rule
The
idea of Kossel – Lewis approach to chemical bond lead to the octet rule, which
states that “the atoms transfer or share electrons so that all atoms involved
in chemical bonding obtain 8 electrons in their outer shell (valence shell)”.
Related Topics