Chemical bonding - Valence Bond Theory | 11th Chemistry : UNIT 10 : Chemical bonding
Chapter: 11th Chemistry : UNIT 10 : Chemical bonding
Valence Bond Theory
Valence
Bond Theory
Heitler
and London gave a theoretical treatment to explain the formation of covalent
bond in hydrogen molecule on the basis of wave mechanics of electrons. It was
further developed by Pauling and Slater. The wave mechanical treatment of VB
theory is beyond the scope of this textbook. A simple qualitative treatment of
VB theory for the formation of hydrogen molecule is discussed below.
Consider
a situation wherein two hydrogen atoms (Ha and Hb) are
separated by infinite distance. At this stage there is no interaction between
these two atoms and the potential energy of this system is arbitrarily taken as
zero. As these two atoms approach each other, in addition to the electrostatic
attractive force between the nucleus and its own electron (purple arrows), the
following new forces begins to operate.
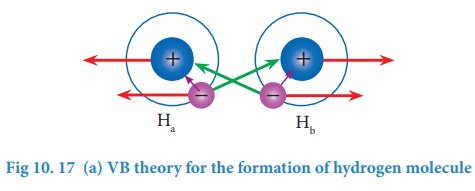
The
new attractive forces (green arrows) arise between
(i)
nucleus of Ha and valence electron of Hb
(ii)
nucleus of Hb and the valence electron of Ha.
The
new repulsive forces (red arrows) arise between
(i)
the nucleus of Ha and Hb
(ii)
valence electrons of Ha and Hb.
The
attractive forces tend to bring Ha and Hb together
whereas the repulsive forces tends to push them apart. At the initial stage, as
the two hydrogen atoms approach each other, the attractive forces are stronger
than the repulsive forces and the potential energy decreases. A stage is
reached where the net attractive forces are exactly balanced by repulsive
forces and the potential energy of the system acquires a minimum energy.
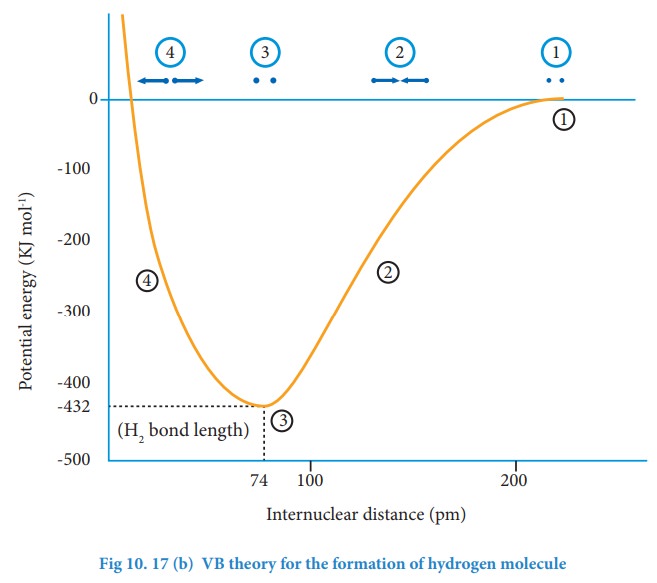
At
this stage, there is a maximum overlap between the atomic orbitals of Ha
and Hb, and the atoms Ha and Hb are now said
to be bonded together by a covalent bond. The internuclear distance at this
stage gives the H-H bond length and is equal to 74 pm. The liberated energy is
436 kJ mol-1 and is known as bond energy. Since the energy is
released during the bond formation, the resultant molecule is more stable. If
the distance between the two atoms is decreased further, the repulsive forces
dominate the attractive forces and the potential energy of the system sharply
increases
Salient features of VB Theory:
(i)
When half filled orbitals of two atoms overlap, a covalent bond will be formed
between them.
(ii)
The resultant overlapping orbital is occupied by the two electrons with
opposite spins. For example, when H2 is formed, the two 1s electrons
of two hydrogen atoms get paired up and occupy the overlapped orbital.
(iii) The strength of a covalent bond depends upon the extent of overlap of atomic orbitals. Greater the overlap, larger is the energy released and stronger will be the bond formed.
(iv) Each atomic orbital has a specific direction (except
s-orbital which is spherical) and hence orbital overlap takes place in the
direction that maximizes overlap
Let
us explain the covalent bond formation in hydrogen, fluorine and hydrogen
fluoride using VB theory.
Related Topics