Chemical bonding | Chemistry - Covalent bonds | 11th Chemistry : UNIT 10 : Chemical bonding
Chapter: 11th Chemistry : UNIT 10 : Chemical bonding
Covalent bonds
Types
of chemical bonds
The
chemical bonds can be classified based on the nature of the interaction between
the bonded atoms. Two major types of chemical bonds are covalent bonds and
ionic bonds. Generally metals reacts with non-metals to form ionic compounds,
and the covalent bonds are present in the compounds formed by nonmetals.
Covalent bonds:
Do
you know all elements (except noble gases) occurs either as compounds or as
polyatomic molecules? Let us consider hydrogen gas in which two hydrogen atoms
bind to give a dihydrogen molecule. Each hydrogen atom has one electron and it
requires one more electron to attain the electronic configuration of the
nearest noble gas helium. Lewis suggested that both hydrogen atoms will attain
the stable configuration by mutually sharing the electrons available with them.
Similarly, in the case of oxygen molecule, both the oxygen atoms share two
electron pairs between them and in nitrogen molecule three electron pairs are
shared between two nitrogen atoms. This type of mutual sharing
of one or more pairs of electrons between two combining atoms results in the
formation of a chemical bond called a covalent bond. If two atoms share
just one pair of electron a single covalent bond is formed as in the
case of hydrogen molecule. If two or three electron pairs are shared between
the two combining atoms, then the covalent bond is called a double bond or a
triple bond, respectively.
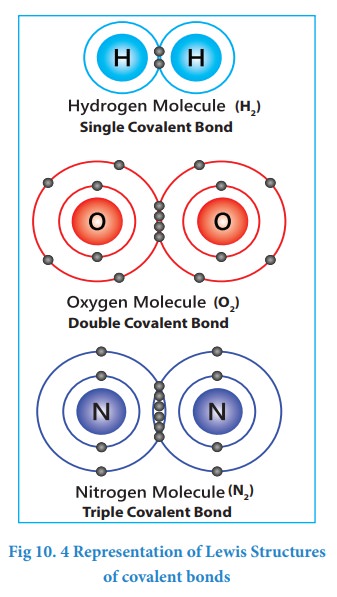
Representing a covalent bond - Lewis structure (Lewis dot structure)
Lewis
structure (Lewis dot structure) is a pictorial representation of covalent
bonding between the combining atoms. In this structure the shared valence
electrons are represented as a pair of dots between the combining atoms and the
unshared electrons of the atoms are represented as a pair of dots (lone pair)
on the respective individual atoms.
The
Lewis dot structure for a given compound can be written by following the steps
given below. Let us understand these steps by writing the Lewis structure for
water.
1. Draw the skeletal structure of
the molecule.
In general, the most electronegative
atom is placed at the centre. Hydrogen and fluorine atoms should be placed at
the terminal positions. For water, the skeletal structure is

2. Calculate the total number of
valence electrons of all the atoms in the molecule. In case of polyatomic ions the charge on ion should also be
considered during the calculation of the total number of valence electrons. In
case of anions the number of negative charges should be added to the number of
valence electrons. For positive ions the total number of positive charges
should be subtracted from the total number of valence electrons.
In
water, total number of valence electron = [2×1 (valence electron of hydrogen)] +
[1 × 6 (valence electrons of oxygen)] = 2 + 6 = 8.
![]()
![]()
3. Draw a single bond between the
atoms in the skeletal structure of the molecule. Each bond will account for two valence electrons (a bond pair).
For water, we can draw two bonds accounting for four valence electrons as
follows.

4. Distribute the remaining valence
electrons as pairs (lone pair), giving octet (only duet for hydrogen) to the
atoms in the molecule.
The distribution of lone pairs
starts with the most electronegative atoms followed by other atoms.
In
case of water, the remaining four electrons (two lone pairs) are placed on the
most electronegative central oxygen, giving octet.
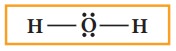
5. Verify weather all the atoms
satisfy the octet rule (for hydrogen duet). If not, use the
lone pairs of electrons to form additional bond to satisfy the octet rule.
In
case of water, oxygen has octet and the hydrogens have duets, hence there is no
need for shifting the lone pairs. The Lewis structure of water is as follows
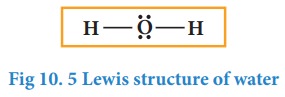
Let
us draw the Lewis structure for nitric acid.
1.
Skeletal structure
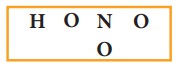
2.
Total number of valence electrons in HNO3
=
[1 × 1(hydrogen)] + [1 × 5(nitrogen)] + [3× 6(oxygen)] = 1+ 5 + 18 = 24
3.
Draw single bonds between atoms. Four bonds can be drawn as shown in the figure
for HNO3 which account for eight electrons (4 bond pairs).
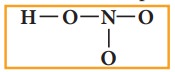
4. Distribute the remaining sixteen (24 - 8= 16) electrons as eight lone pairs starting from most electronegative atom, the oxygen. Six lone pairs are distributed to the two terminal oxygens (three each) to satisfy their octet and two pairs are distributed to the oxygen that is connected to hydrogen to satisfy its octet.
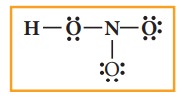
5.
Verify weather all the atoms have octet configuration. In the above
distribution, the nitrogen has one pair short for octet. Therefore, move one of
the lone pair from the terminal oxygen to form another bond with nitrogen.
The
Lewis structure of nitric acid is given as
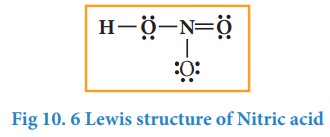
Formal charge:
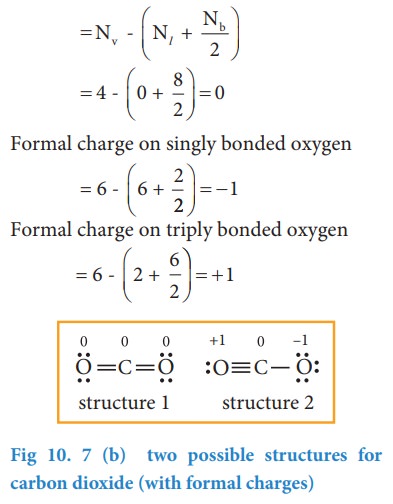
Let
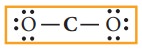
us draw the Lewis structure for carbon dioxide.
1.
Skeletal structure

2.
Total number of valence electrons in CO2
=[1
x 4(carbon)] +[2 x 6(oxygen)] = 4+ 12 = 16
3.
Draw single bonds between atoms. Two bonds can be drawn as shown in the figure
for CO2 which accounts for four electrons (2 bond pairs).

4.
Distribute the remaining twelve electrons (16 - 4= 12) as six lone pairs
starting from most electronegative atom, the oxygen. Six lone pairs are
distributed to the two terminal oxygens (three each) to satisfy their octet.
5.
Verify weather all the atoms have octet configuration. In the above
distribution, the central carbon has two pair short for octet. Therefore, to
satisfy the octet rule two lone pairs from one oxygen or one pair from each
oxygen can be moved to form multiple bonds, leading the formation of two
possible structures for carbon dioxide as shown below
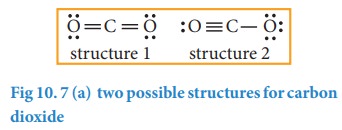
Similarly,
the Lewis structure for many molecules drawn using the above steps gives more
than one acceptable structure. Let us consider the above mentioned two
structures of carbon dioxide.
Which
one the above forms represents the best distribution of electrons in the
molecule. To find an answer, we need to know the formal charge of each atom in
the Lewis structures. Formal charge of an atom in a molecule, is the electrical
charge difference between the valence electron in an isolated atom and the
number of electrons assigned to that atom in the Lewis structure.
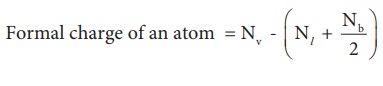
Where,
Nv- Number of valence electron of atom in its
isolated state.
Nl - Number of electrons present as lone pairs around the atom in the
Lewis structure
Nb
- Number of electrons present in bonds
around the atom (bond pairs) in the Lewis structure]
Now
let us calculate the formal charge on all atoms in both structures,
For
Structure 1,
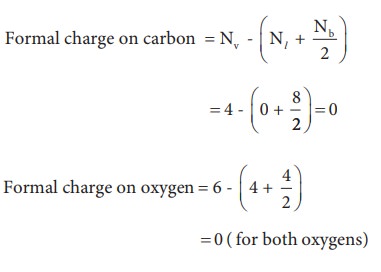
For structure 2
Formal charge on carbon
After
calculating the formal charges, the best representation of Lewis structure can
be selected by using following guidelines.
1.
A structure in which all formal charges are zero preferred over the one with
charges.
2.
A structure with small formal charges is preferred over the one with higher
formal charges.
3.
A structure in which negative formal charges are placed on the most
electronegative atom is preferred.
![]()
![]() In case of CO2 structures, the structure one is
preferred over the structure 2 as it has zero formal charges for all atoms.
In case of CO2 structures, the structure one is
preferred over the structure 2 as it has zero formal charges for all atoms.
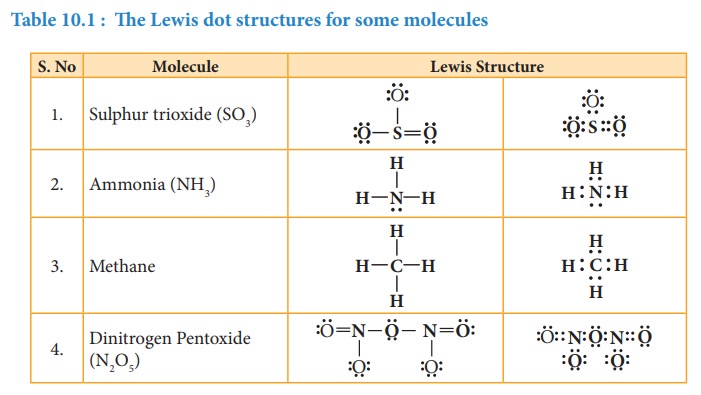
Lewis structures for exceptions to octet rule
The
octet rule is useful for writing Lewis structures for molecules with second
period element as central atoms. In some molecules, the central atoms have less
than eight electrons around them while some others have more than eight
electrons. Exception to the octet rule can be categorized into following three
types.
1.
Molecules with electron deficient central atoms
2.
Molecules containing odd electrons
3.
Molecules with expanded valence shells
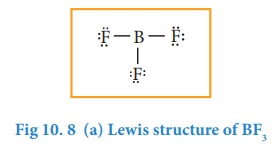
1. Molecules with electron deficient central atoms
Let
us consider boron trifluoride, as an example. The central atom boron has three
valence electron and each fluorine has seven valence electrons. The Lewis
structure is
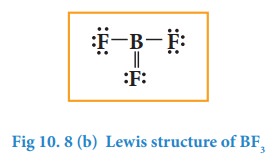
In the
above structure, only
six electrons around boron atom. Moving a lone pair from one of the
fluorine to form additional bond as shown below.
However,
the above structure is unfavourable as the most electronegative atom fluorine
shows positive formal charge and hence the structure with incomplete octet is
the favourable one. Molecules such as BCl3, BeCl2, etc...
also have incomplete octets.
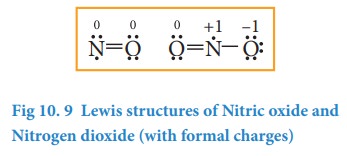
2. Molecules containing odd electrons
Few
molecules have a central atom with an odd number of valence electrons. For
example, in nitrogen dioxide and nitric oxide all the atoms does not have octet
configuration. The lewis structure of the above molecules are shown in the
figure.
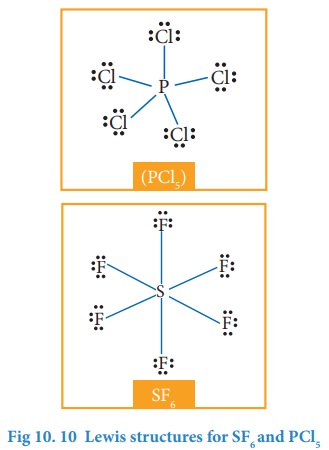
3. Molecules with expanded valence shells
In
molecules such as sulphur hexafluoride (SF 6), phosphorous
pentachloride (PCl5) the central atom has more than eight valence
electrons around them. Here the central atom can accommodate additional electron
pairs by using outer vacant d orbitals. In SF6 the central atom
sulphur is surrounded by six bonding pair of electrons or twelve electrons.
Related Topics